
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
This book, cohesively written by an expert author with supreme breadth and depth of perspective on polyurethanes, provides a comprehensive overview of all aspects of the science and technology on one of the most commonly produced plastics.
- Covers the applications, manufacture, and markets for polyurethanes, and discusses analytical methods, reaction mechanisms, morphology, and synthetic routes
- Provides an up-to-date view of the current markets and trend analysis based on patent activity and updates chapters to include new research
- Includes two new chapters on PU recycling and PU hybrids, covering the opportunities and challenges in both
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Edition
2Subtopic
Industrial & Technical Chemistry1
INTRODUCTION
In the early 1900s there were very few of the synthetic polymers we have grown accustomed to now. During succeeding years polymer science experienced explosive growth with the invention of polyvinyl chloride (PVC, 1913), polyethylene (1933), polyvinylidene chloride (Saran, 1933), polyamides (nylon, 1934), and polytetrafluoroethylene (Teflon, 1938). In addition, during the 1930s the polymer family known as polyurethanes was invented. Now, of course, polyurethanes, and all the polymers developed during this period, have become an integral part of modern life. As you read this you may not be aware of how many ways polyurethanes surround you. They are present in the shoes you stand in, the seat cushion you sit upon, the carpet backing and foam pad underlay you walk upon, in the fibers of your clothing, insulation of your walls and roof, in your refrigerator, dishwasher, water heater, automotive seating, automotive structural foam, automotive paints and coatings, furniture coatings, your bed mattress, the adhesive holding this book together – the list just goes on. This book’s purpose is to explain polyurethane science, technology, applications, trends, and markets in virtually all of its forms and relate those structures to the properties that make them so suited for so many uses. It is not an overstatement to say that if polyurethanes are not the most versatile class of materials, then they are certainly one of the most versatile polymer categories in existence.
Discovery of polyurethane chemistry is attributed to the efforts of Otto Bayer and the research team he led at the now defunct I.G. Farben AG chemical company. The first patent associated with polyurethanes was filed in 1937 and numerous other patents, most notably the production of flexible foams resulting from isocyanate–water reactions, were filed thereafter. I.G. Farben was broken up following World War II for complicity in war crimes and the company’s top leaders were convicted of crimes against humanity (exploitation of slave labor and production of nerve gas). The largest surviving components of I.G. Farben – Bayer AG and BASF SE – remain very large and respected global industrial concerns. While BASF continues to engage with and maintain a significant presence within the polyurethane industry, Bayer spun off its polyurethane business and the rest of its industrial chemicals concerns into a new company called Covestro.
After the initial discovery and expositions of basic chemistry, mostly based on short‐chain diols and polyester polyols, industrial polyurethanes saw immense growth following the development of polyether polyols by E.I. du Pont de Nemours and Company (now known as DuPont) and The Dow Chemical Company. While Dow Chemical remains one of the world’s largest manufacturers of polyurethane chemicals, DuPont has exited its polyurethanes businesses, which were primarily textile and coatings related. While polyesters remain prominent components of polyurethane chemistry, it was the superior processing, low‐temperature flexibility, and hydrolytic stability of polyether polyols that expanded polyurethane polymers into their current acceptance in every aspect of modern life.
As ubiquitous as polyurethanes are, it is perhaps surprising that they represent a relatively minor (but still significant) fraction of the overall global consumption of plastics by volume (Figure 1.1).
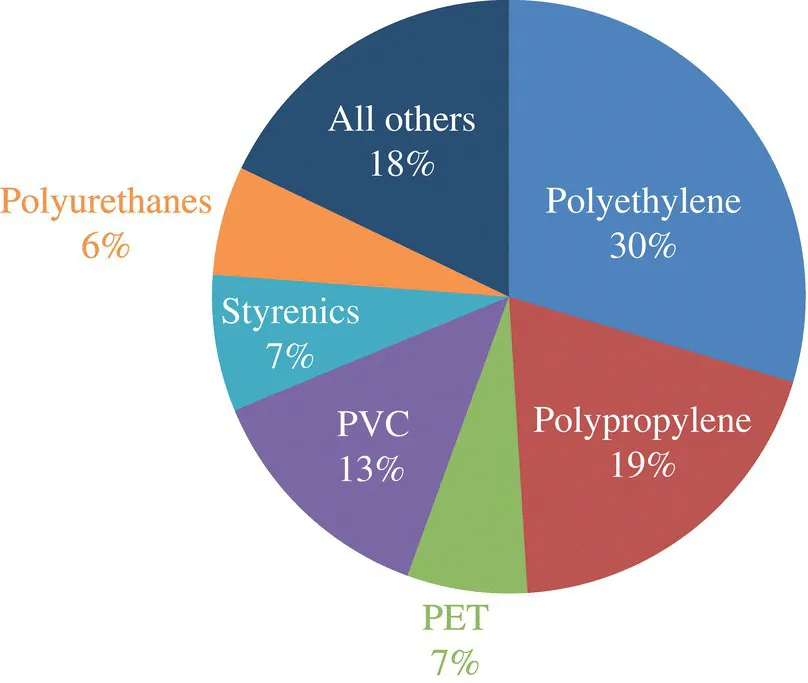
FIGURE 1.1 Percentage global consumption of plastics in 2018. Polyethylene encompasses all densities; styrenics includes all copolymers along with atactic polystyrene. These relative values are similar to those in the first edition using 2012 data. The consumption of many plastics grows at a rate relative to economic activity plus a small accelerator or decelerator for each given plastic’s role in the market. PET = polyethyene terephthate.
The structures of the listed commodity polymers are relatively simple repeating units (Figure 1.2). Their simplicity is in part responsible for their high level of utility and low‐cost positions. The plastics industry has generated variants of the structures shown in Figure 1.2 by, for instance, introducing branches, but these complexities do not fundamentally alter the basic polymer structure.
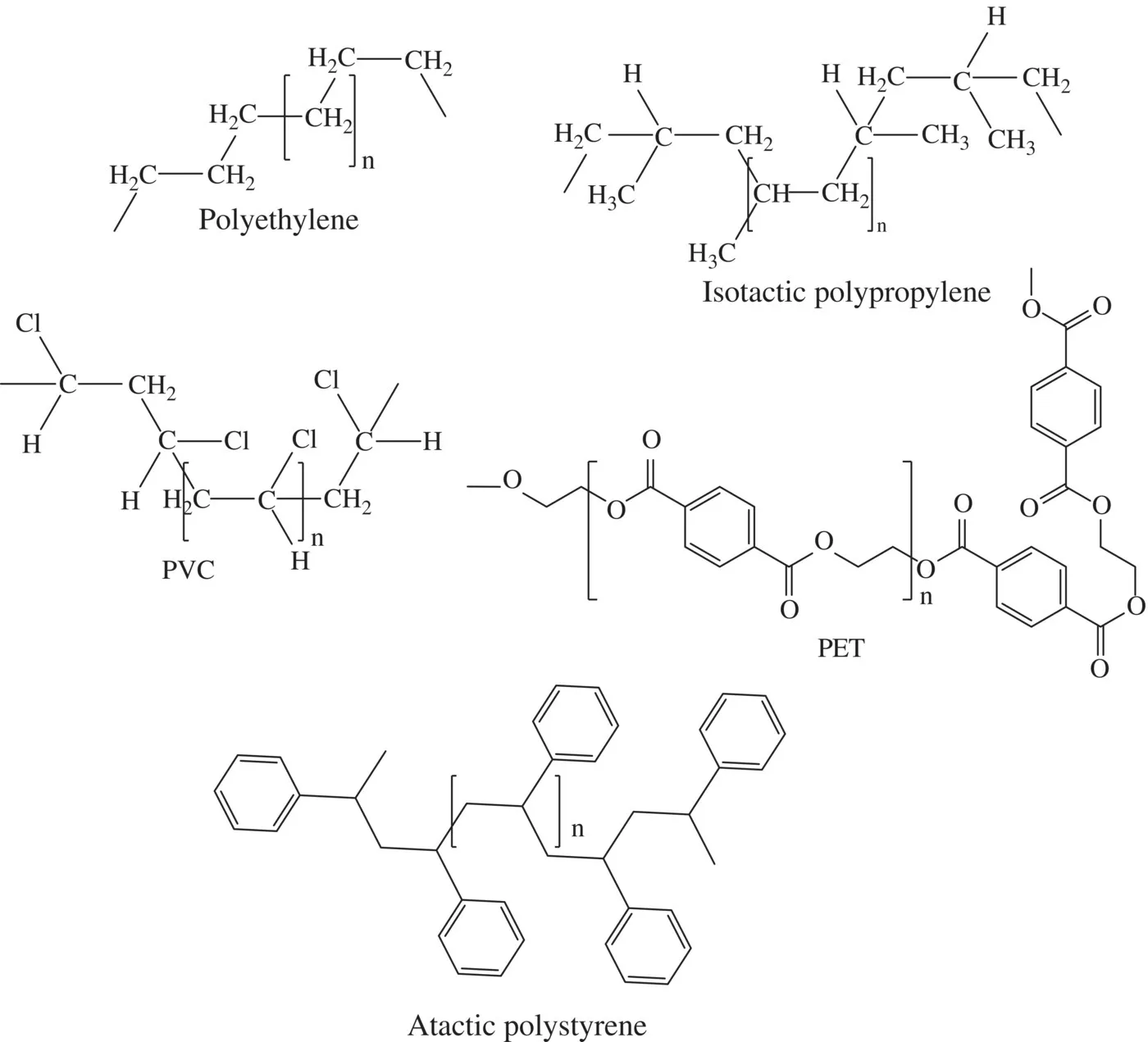
FIGURE 1.2 Illustrative structures of high‐volume commodity polymers.
Polyurethane is the largest volume commodity polymer that cannot be characterized by a simple structure such as that shown in Figure 1.2. Instead, polyurethane represents a class of polymers, and any polymer with a urethane repeat unit is classified as a polyurethane regardless of the other functional or polymer structures incorporated (Figure 1.3).
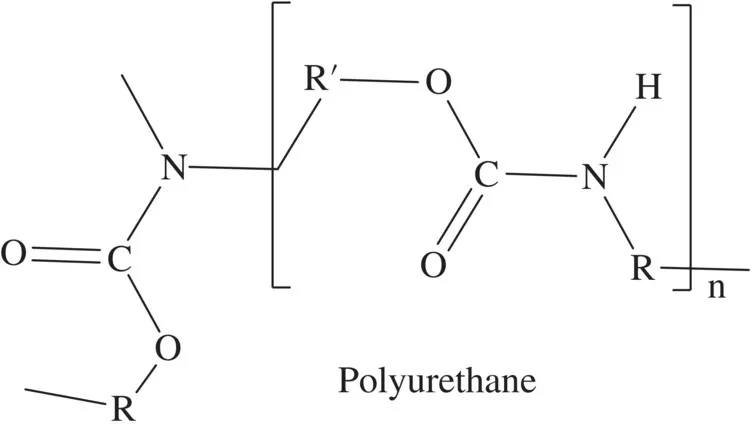
FIGURE 1.3 The urethane unit within a polyurethane polymer chain.
Specific polyurethane structures used for making mattress foam, insulation foam, or shoe foam can be significantly different from one another and cannot be neatly represented like the structures in Figure 1.2. In fact, even structures of different insulation foams can vary so widely that they also cannot be easily represented by a single structure. Another difference with other commodity polymers is that large‐volume polyurethane applications require the mixing of two reactive liquid components rather than the processing of a pellet into a molded or extruded object. Given these complexities it is remarkable that polyurethanes have developed into a commodity plastic category, and it is testament to the versatility and performance of polyurethanes that they are so difficult to replace in their favored applications.
Polyurethane polymers as a class are made from commodity building block reagents and short‐chain polymers (or oligomers). These building blocks include, for example, the following categories: polyisocyanates, polyethers, polyesters, water, and amines (Figure 1.4). As building block categories they also cannot be represented by unique structures and are denoted by “R” to allow designers to insert any conceivable chemically allowable unit.
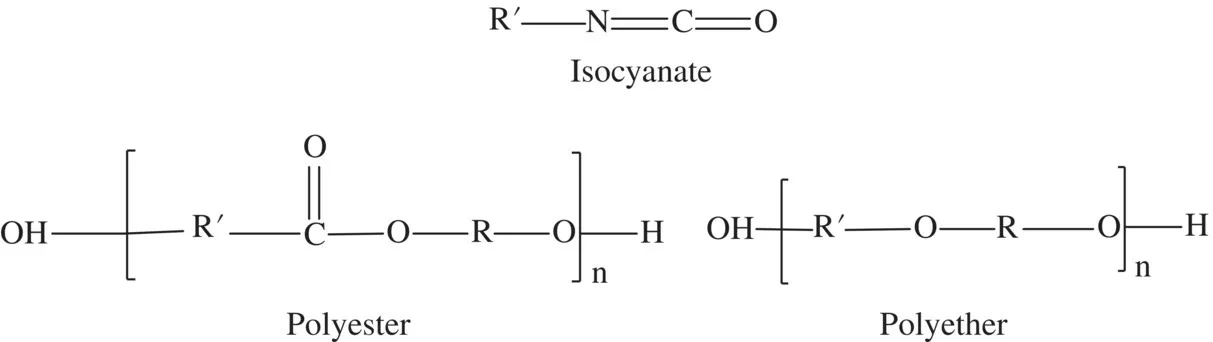
FIGURE 1.4 Chemical structures of isocyanate, polyester, and polyether. To make a polyurethane the Rʹ of the isocyanate structure must also have an isocyanate function [1].
The polyurethane unit is easily mistaken for the related polyester, polyurea, or polyamide (nylon) structures (Figure 1.5). In fact, polyureas, polyesters, and polyurethanes are often joined into polyurethane materials and still broadly classified as polyurethane. (Polyamides were not previously a part of polyurethane chemistry because of their vastly different processing characteristics. However, recent literature indicates nascent explorations of urethane–amide hybrids; see Chapter 13.)
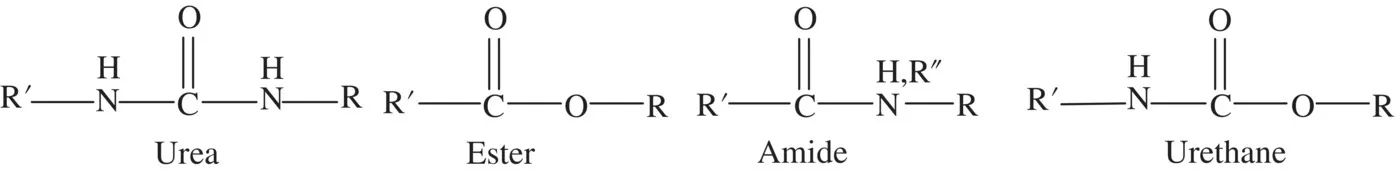
FIGURE 1.5 Structures of urea, ester, amide, and urethane functionalities.
As commodity products, polyurethanes have achieved a certain establishment status in academic ...
Table of contents
- COVER
- TABLE OF CONTENTS
- TITLE PAGE
- COPYRIGHT PAGE
- DEDICATION PAGE
- PREFACE
- ACKNOWLEDGMENTS
- 1 INTRODUCTION
- 2 POLYURETHANE BUILDING BLOCKS
- 3 INTRODUCTION TO POLYURETHANE CHEMISTRY
- 4 THEORETICAL CONCEPTS AND TECHNIQUES IN POLYURETHANE SCIENCE
- 5 ANALYTICAL CHARACTERIZATION OF POLYURETHANES
- 6 POLYURETHANE FLEXIBLE FOAMS
- 7 POLYURETHANE FLEXIBLE FOAMS
- 8 POLYURETHANE RIGID FOAMS
- 9 POLYURETHANE ELASTOMERS
- 10 POLYURETHANE ADHESIVES AND COATINGS
- 11 SPECIAL TOPIC
- 12 SPECIAL TOPIC
- 13 POLYURETHANE HYBRID POLYMERS
- 14 RECYCLING OF POLYURETHANES
- INDEX
- END USER LICENSE AGREEMENT
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Polyurethanes by Mark F. Sonnenschein in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Industrial & Technical Chemistry. We have over one million books available in our catalogue for you to explore.