![]()
PART 1
Materials
![]()
CHAPTER 1
Defects with materials
1.1 Material properties generally
Learning objectives
- You will be made aware of a range of physical properties of common building materials.
- You should recognize that these properties are essential features in the understanding of deficiency in buildings.
Material properties generally
The properties of materials shown in Table 1.1 are for general guidance only. There are specific properties for particular materials and further information can be obtained from the ‘Further reading’ list.
The ‘melting points’ given in Table 1.1 for GRP, unplasticized PVC and plasticized PVC are, strictly, inappropriate. This is also true for glass, which does not have a well-defined melting point. The temperature shown is where the material starts to move as a fluid.
Although reversible shrinkage has been tabulated here, several materials also exhibit irreversible shrinkage.
Where there is a dash in the table this generally indicates that within the general description of the material there are a variety of different forms available. This in turn means that there are a wide range of published values for this material. The properties of timber are described in section 4.1 of this book.
Discussion topics
- Describe the influence of physical factors on the deterioration of three building materials.
- Discuss the use of the term ‘lack of strength’ to describe the deterioration of building materials.
- Explain the anisotropic nature of timber.
- Compare the influence of chemical properties on the deterioration of ferrous metal and aluminium.
- ‘Because modern construction makes use of high-quality materials the incidence of defects will inevitably decline’. Discuss.
Table 1.1 General properties of common building materials
Further reading
Cook, G.K. and Hinks, A.J. (1992) Appraising Building Defects: Perspectives on Stability and Hygrothermal Performance, Longman Scientific & Technical, London.
Curwell, S.R. and March, C.G. (eds) (1986) Hazardous Building Materials: A Guide to the Selection of Alternatives, E. & F.N. Spon, London.
Desch, H.E. (1981) Timber: Its Structure, Properties and Utilisation, 6th ed (revised by J.M. Dinwoodie), Macmillan Education, London.
Everett A. (1975) Materials—Mitchells Building Construction, Longman Scientific & Technical London.
Richardson, B.A. (1991) Defects and Deterioration in Buildings, E. & F.N. Spon, London.
Taylor, G.D. (1991) Construction Materials, Longman Scientific & Technical, London.
1.2 Stone
Learning objectives
You should be able to:
- compare the influence of moisture and thermal movement on the durability of stones used in construction;
- explain the methodology of chemical deterioration of building stones;
- compare the chemical deterioration of different building stones;
- describe the influence of porosity on the degree of exposure permitted for limestones;
- describe the influence of cleavage planes on the durability of slate, shale and sandstone.
Types of stone can be classified with respect to age, composition, behaviour and location. In general the difference associated with age is the accepted method of describing building stones. There are a large variety of building stones used in construction; these include igneous, sedimentary and metamorphic stones.
Deterioration of igneous stone
This stone is produced by cooling of fluid from the interior of the earth. This ‘magma’ can be 500 to 600 million years old or may be more recently formed from volcanoes. This cooling may be in the air (extrusive igneous rocks), or underground (the ‘intrusive’ or ‘plutonic’ igneous rocks). These rocks cool more slowly and are generally more crystalline, e.g. granites.
Igneous stone can be hard, durable, impermeable and inert. Some basalts and dolerites have significant moisture movement characteristics causing a moisture movement coefficient of 0.1% to 0.3% in concrete. The deterioration mechanisms associated with igneous rocks are commonly associated with brittle failure or surface discoloration caused by weathering or condensation. They are commonly of a minor nature.
Sedimentary stone—general
The compaction of weathered igneous rocks or shell fragments produces sedimentary rocks. In general the compacted shell fragments produce limestones and the weathered igneous rocks produce sandstones.
Problems with limestones
The limestones, which are very common in the UK, are predominantly composed of calcium carbonate. Other compounds, e.g. magnesium carbonate, may be present and these give particular characteristics to the limestone. Porosity can vary between 1% and 40%. This and the saturation coefficient are coarse measures of durability, which for limestones can be more accurately assessed by quantifying the percentage of pores below 5 microns, where<30%=durable, >90%=not durable. A crystallization test can be carried out on limestone samples. They are soaked in a sodium sulphate solution and dried 15 times, and the resultant effects are used to classify the stone on a six-point scale from ‘A’ to ‘F’. These are used to define appropriate exposure zones and can be used to identify inappropriate applications.
Table 1.2 BRE exposure zones for limestones
Limestone is chemically more active than sandstone. The quarrying of limestone, which contains a mineral- and salt-rich moisture, may be followed by ‘seasoning’, where the moisture moves to the surface. Evaporation of the water leaves a crystalline crust which is removed when the stone is ‘dressed’. Dressing freshly quarried stone is considered to be easier, although the crystallization will now occur on the finished stone. In general the lower the moisture content of the stone then the greater the frost resistance.
Limestone can deteriorate when exposed to acidic rainwater or any other sulphurous source. Carbon dioxide when dissolved in rainwater has a pH of around 5.6, whereas acid rain has a pH of less than 5. The sulphurous acids can combine with the calcium carbonate to produce calcium sulphate and calcium nitrate in the surface region of the stone. Whilst this may be hard and dense it has different physical characteristics from the base stone and the resultant differential moisture and thermal movement, together with the stresses associated with its crystallization, can cause the stone face to break down. In addition calcium sulphate is slightly soluble, causing the gradual erosion of the stone surface under the action of rainwater. The calcium nitrate is hygroscopic and will absorb water from the air. This will drive the deterioration process and accelerate the deterioration of the stone.
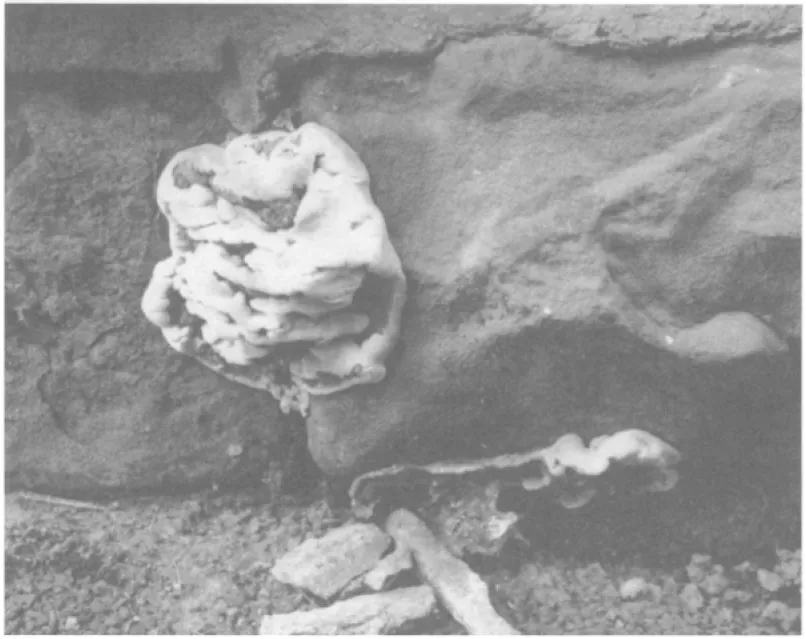
Fig. 1.1. The possible organic attack of stonework or of other material behind the stonework. Fruiting bodies may develop away from the direct region of attack. (S.D.McGlynn.)
There are also problems associated with the bacteria, algae, fungi and lichens commonly present on the stone surface. Some of the bacteria have the ability to convert the sulphurous and nitrous acids from environmental pollution to sulphuric and nitric acids, which can be more damaging to the stone.
Water run-off from limestone can cause pattern staining of façades and may also cause deterioration of sandstone. Additional staining may come from chemicals washed from adjacent materials, e.g. green staining from copper.
Problems with sandstones
The sandstones are commonly held together with a silica or calcium matrix, in well-defined bedding planes of differing composition. Where quartz is held together with a silica matrix this is termed silicaeous sandstone; with a calcium carbonate matrix, calcareous sandstone; or where calcium carbonate and magnesium carbonate form the matrix, these rocks are termed dolomitic sandstones.
The porosity of these sandstones can vary between 1% and 25% and saturation coefficients between 0.5 and 0.7. Since this variation can occur within similar sandstones the weathering performance and frost resistance of the stone may vary, even on the same building.
These stones may also be damaged because of crystallization of calcium sulphate or calcium nitrate within the stone and below the surface. Atmospheric pollution can cause deterioration of sandstone when the sulphurous gases in the air condense to react with the calcium carbonate, producing soluble calcium sulphate. The attack mechanism is similar to that which affects limestones. Water run-off from limestone can cause contour scaling of a sandstone surface. The water run-off will enrich the sandstone surface and underlying layers with calcium sulphate and calcium nitrate. The calcium sulphate and calcium nitrate enrichment of the surface crust causes differential thermal and moisture movement problems. This can result in the surface becoming detached in a manner which follows the contours of the stone surface.
In external applications delamination can occur where bedding planes have been laid vertically, parallel to the building façade. Where the bedding planes are laid horizontally in highly carved stones, there is a risk of localized deterioration where the weathering of exposed and unrestrained bedding planes can occur.
Dissolved salts from groundwater and those from sea spray can cause disruptive damage to limestones and sandstones. Where sodium chloride or sodium sulphate has crystallized the effects are likely to be widespread across the surface of the stone.
Problems with metamorphic stone
The modification of rocks or other material by heat and pressure can produce hard, durable and attractive rocks. These are termed metamorphic rocks and may also be composed of older igneous rocks, e.g. granites. This rock classification includes the slates, which are formed from clay, and marble, which is formed from calcium-rich rocks such as limestone and sandstone. Low compaction, as in the case of shales, can lead to a likelihood of moisture movement, which, where cleavage pla...