
This is a test
- 221 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Basics of Ecotoxicology
Book details
Book preview
Table of contents
Citations
About This Book
This textbook presents a comprehensive examination of environmental science and ecotoxicology for undergraduate students. The material provides sufficient related background information leading to a competency to clearly understand ecotoxicology concepts and topics.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Basics of Ecotoxicology by Donald W. Sparling in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Chemistry. We have over one million books available in our catalogue for you to explore.
Section II
Major Groups of Contaminants: Where They Come from, What They Can Do
4
Metals
4.1Introduction
In the next few chapters, we will study organic contaminants, those that are mainly man-made and have carbon as their principal constituent. Most of these compounds were specifically designed for their ability to either kill or repel organisms. In this chapter, we discuss a very different type of contaminant that is both inorganic and, in some cases, actually required for life. These are the elements collectively called metals that occupy most of the Periodic Chart of Elements (see Figure 2.2).
Given the number of elements that belong to the metals group, you can imagine that there is a wide variety in their chemical behaviors. Metals at the far left of the Periodic Chart are highly soluble in water to the point that they need to be protected from water. For instance, sodium is highly explosive when it comes in contact with water. As we progress to the right of the chart, we encounter metals that are soluble in slightly acidic water to those that are only soluble in very acidic solutions. Mercury does not dissolve in water, although a temporary suspension can be made with this heavy metal. Metalloids share some but not all of the properties of metals, and those of major interest to the ecotoxicologist include boron (B), selenium (Se), germanium (Ge), arsenic (As), actinium (Ac), tellurium (Te), and polonium (Po).
Perhaps this is a good place to clarify some terms. Environmental chemicals including contaminants are taken up into the body or assimilated when they become incorporated into tissues. When chemicals leave the body through urination, defecation, or respiration, or are broken down into simpler compounds that leave the organism, they are said to have been depurated. Some contaminants including heavy metals may have higher rates of assimilation than depuration and thus bioconcentrate, meaning that the concentration of a particular compound is higher than in the environment. If the concentrations of a compound increase up the food chain due to transfer from one level to another, they are said to biomagnify. Except for mercury or when they are combined with organic molecules and become organometals, heavy metals seldom, if ever, biomagnify. Some contaminants including organometals are both lipophilic and hydrophobic, however, in that they concentrate in fats within organisms and do not mix well with water. In contrast, other molecules are both lipophobic and hydrophilic if they avoid lipids and mix well or dissolve readily in water.
4.2Sources of Metals in the Environment
The lithosphere or terrestrial environment is the principal reservoir of metals (Figure 4.1). Metals are located at all levels of the earth, from surface soils to magma in its core. Those close to the surface can be blown around in the atmosphere or washed into bodies of water in runoff. Earthquakes, upheavals, and especially volcanoes bring metals from deep within the earth to the crust. Metals in the atmosphere may be carried to remote areas until they are deposited back onto land or water. Metals in water may be deposited into sediments for eons or carried to oceans.
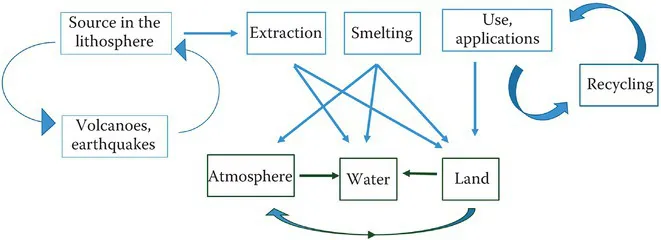
FIGURE 4.1 Major sources of metals in the earth and how the different sources interact.
Metals with economic value are mined and smelted to produce purer forms of the raw substance. These activities have been principal anthropogenic sources of metals in the environment. Other sources of environmental contamination occur whenever metals are used in industrial applications. Prior to catalytic converters, automobile exhausts were also significant sources of lead, but today catalytic converters require unleaded gasoline. Combustion of coal can also be a major source of metal pollution into the atmosphere. Such pollution continues today but many developed countries have strict regulations to reduce the output of these metals by requiring smokestack scrubbers and other technologies to clean the exhausts before they leave the stack. Many developing countries do not have such regulations, and metal pollution remains a global problem.
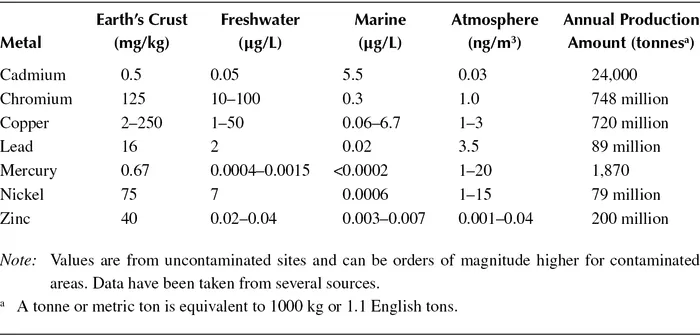
Just where metals are distributed and how much depends, of course, on the metal. Table 4.1 provides some concentration data for major environmental compartments and a handful of metals. As you can see, the earth’s crust has the highest concentrations. This is followed by water—either fresh or marine—and finally by air. The table also includes data on the annual extraction and refinement of these metals. As might be expected, copper and chromium lead the list in this category, but neither of these even compare to the big production metals such as aluminum or iron, which are extracted at the trillions of tonnes level.
TABLE 4.1
Concentrations of a Few Heavy Metals in Various Environmental Compartments along with Annual Production Amounts
Concentrations of a Few Heavy Metals in Various Environmental Compartments along with Annual Production Amounts
4.3Factors Affecting the Behavior of Metals
Metals can occur in their inorganic forms either as ions or as nonionic elements. They can also combine with organic molecules such as ethyl or methyl groups.
The acidity of the environment, as measured by pH, is a major factor in determining the valence state of a metal. Metals are oxidized at low pH, that is, they move from a low ionization state...
Table of contents
- Cover
- Halftitle Page
- Title Page
- Copyright Page
- Contents
- Preface
- Acknowledgments
- Author
- SECTION I An Introduction to Ecotoxicology
- SECTION II Major Groups of Contaminants: Where They Come from, What They Can Do
- SECTION III Higher Level Effects, Analysis of Risk, and Regulation of Chemicals
- Glossary
- Index