![]()
Chapter 1
Atoms, Elements and Compounds
Contents
- Starting small
- Atoms
- Elements
- Neutrons
- Stable or unstable?
- Compounds
- Covalent and hydrogen bonding
- Ionic bonding
- Ions and electrolytes
- Diffusion
- Osmosis
- pH
- Metabolism
- Fluid compartments
- Fluid balance
- Chapter 1: Test yourself
Starting Small
When thinking about how the human body works most of us tend to consider the familiar structures of the heart, lungs, kidneys, etc. We faintly remember from school that the heart is a muscular pump, the lungs allow us to breathe and the kidneys produce urine (but how any of this actually happens is a distant memory). Whilst these and other organs are self-evidently of great importance for our health, we should not overlook the much smaller and equally important structures that collectively make up these well-known parts of the human body. For example, the smallest functional unit of the body is the cell and it is estimated that the average adult possesses about 30 trillion (30,000,000,000,000) of these. However, even these microscopic units are composed of even smaller structures that are constantly engaged in the breakdown and production of chemicals and compounds necessary to maintain cellular and overall health. I know what you are thinking – ‘this already sounds boring’ – but hopefully I can convince you that the small as well as the big is worth knowing about and even quite interesting. In order to understand how the body works we first need to look at the simplest level of organisational structure – the atom. The way in which these fickle and impulsive particles bond and disassociate with one another enables the body to function in the manner that it does. I am perhaps being a little unfair to all atoms since some of them possess relatively calm and settled dispositions. However, it is also fair to say that many have personality disorders that rival the average Big Brother contestant and some are extremely (and usefully) unstable.
Atoms
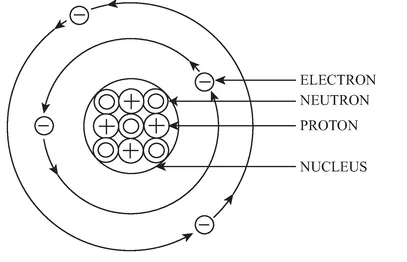
The word atom is derived from an idea first formulated by the ancient Greek philosopher Democritus over 2,000 years ago. Democritus believed that everything was made from indivisible, imperishable and unchanging particles which he called atamos. A remarkable and insightful deduction for someone with no modern scientific equipment at his disposal. Today, atoms are often described as the smallest unit of matter that can exist in a stable form. They are made up of a number of smaller subatomic (literally ‘below’ or ‘less than’ atomic) particles that behave in a much more unpredictable fashion. However, for the purposes of this book, we are only interested in three of these particles: protons, neutrons and electrons. Each atom has a central core called a nucleus which contains varying numbers of protons and neutrons. The nucleus is surrounded by one or more energy layers called a shell which contains the electrons (Figure 1.1). Protons are positively charged particles, neutrons have no charge (they are neutral) and electrons are negatively charged. This is important since, under ‘normal’ circumstances, the number of protons in the atom’s nucleus is equal to the number of electrons in the atom’s outer shell (or shells). This ensures that the atom remains electrically neutral since the positive charge of the proton is cancelled out by the negative charge of the electron. It doesn’t matter how many neutrons there are in the nucleus of the atom (in terms of electrical charge) since they are always neutral.
Figure 1.1 Basic structure of an atom.
Elements
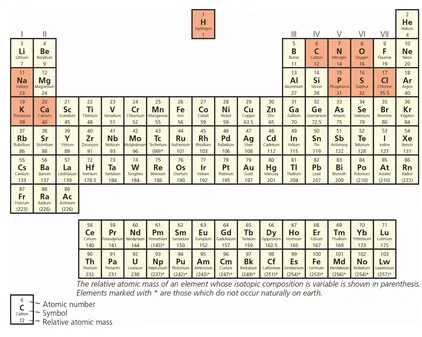
Figure 1.2 Periodic table of elements. Ninety-nine percent of the human body is composed of those highlighted in orange.
Do you remember the periodic table of elements from school? Every physics and chemistry classroom had one of these impressively large charts on the wall somewhere. Each of the elements represented on the periodic table is composed of atoms of the same type (Figure 1.2). That is to say, each element is composed of either a single atom or a number of atoms of the same type. For example, an atom of hydrogen (chemical symbol H) represents the element hydrogen. If we add another hydrogen atom (H2) it remains the element hydrogen but can also be described as a molecule of hydrogen. The term molecule can be confusing since it is applied when two or more atoms form chemical bonds with each other. However, in the case of a molecule, it doesn’t matter if the atoms are the same or different from each other (unlike a compound which refers to the combination of two or more DIFFERENT atoms). I chose hydrogen as an example simply because it is the first element of the periodic table and has the atomic number 1. Did you ever wonder why hydrogen receives this pre-eminent position in the league table of elements? Why should hydrogen be number 1 when gold (Au) is languishing at number 79 and platinum (Pt) is only doing slightly better at 78? The answer is very simple and relates to the number of protons contained in the atom’s nucleus. Hydrogen has the atomic number 1 because it has one proton in its nucleus. Gold has the atomic number 79 because it has 79 protons in its nucleus. Oxygen (O) has the atomic number 8 because, you guessed it, it has eight protons in its nucleus. The fundamental difference between lead (Pb, atomic number 82) and gold (Au, atomic number 79) is the number of protons in the nucleus. However, as any good alchemist will tell you, it turns out to be incredibly tricky converting one into the other.
Neutrons
Neutrons are also found in the nucleus . . . but what do they actually do? We know that the number of neutrons in the nucleus does not affect the electrical charge of the atom since they are always uncharged particles. They do, however, help to determine the mass number of the atom. Mass simply means how much matter a solid, liquid or gas contains. You are probably thinking: ‘Isn’t that the same as weight?’ Well, yes and no. For example, the mass of a small gold bar may be 2 kilograms (a unit of weight). Mass is commonly measured by how much something weighs but, crucially, weight can change depending on where you happen to be at the time. Mass, on the other hand, will always remain the same. For example, if we weighed the same gold bar on the moon, it would weigh less than it does on earth but still have the same mass. Following this argument to its logical conclusion: if you wish to lose weight, without going on a diet, simply move to the moon or other low-gravity environment. Anyway, what has this got to do with neutrons? The atomic mass number is the total of the number of protons and the number of neutrons in the nucleus of the atom. Helium, for example, has two protons and two neutrons in its nucleus. Consequently, it has an atomic number of 2 and an atomic mass number of 4. Just to confuse matters, not all atoms of an element have the same number of neutrons in their nucleus. We refer to these atoms as isotopes since they have a different atomic mass. Atomic weight (or relative atomic mass) is slightly different. It is calculated by taking an average (mean) of the relative atomic mass numbers of all the isotopes for a particular element. However, I feel this is getting unnecessarily complicated. The bottom line is that protons + neutrons = the mass number of the atom.
Stable or Unstable?
The final constituent of the atom is the electron. These, as you remember, are negatively charged particles that whip around the nucleus of the atom in shells of varying size. The first or innermost shell can house a maximum of two electrons. The second shell can accommodate a maximum of eight electrons. The third shell is a little more complicated, and the first 20 elements (hydrogen to calcium) possess outer/third shells that hold a maximum of eight electrons. After this, the outer/third shell electron numbers become more variable and iron, for example, has 14 electrons in its third shell (2, 8, 14, 2) whilst copper (and all elements following it) have 18. Anyway, the point is this: elements are much like us in that they are constantly searching for harmony and inner peace in their lives. In order to achieve this seemingly impossible goal all they have to do is completely fill their outer electron shell. To put it another way, atoms with an incomplete outer shell are said to be chemically active since they are looking to combine (or bond) with another atom’s electrons. Those that have already achieved this Nirvana-like state of completeness (e.g. those with a full outer electron shell) are referred to as chemically inactive or stable. The best example of this phenomenon is provided by the first two elements of the periodic table: hydrogen (H) and helium (He). Hydrogen has one proton in its nucleus and one electron in its first (and only) shell. It has one spare space, therefore, and is constantly on the look-out for an electron to fill this yawning gap in its life. Hydrogen is definitely an unstable (or chemically active) atom. If you don’t believe me just consider the hydrogen bomb. Helium, on the other hand, has two protons in its nucleus and two electrons in its first (and only) shell. In contrast to hydrogen, therefore, helium is a very easy-going atom and forms one of a very exclusive group of elements who can boast a complete outer electron shell. These elements are known as the noble (or inert) gases and occupy the right-hand column of the periodic table (atomic numbers: 2, 10, 18, 36, 54, 86). Run 15,000 volts of electricity through neon whilst in a sealed glass tube and it just glows a pretty colour (irritatingly smug). Another good example of the difference in the personality between hydrogen and helium is, and I am slightly embarrassed to say this, airships. Let me explain before you throw this book away in exasperation. On 6 May 1937, the German airship Hindenburg burst into flames whilst attempting to land in New Jersey, killing 36 passengers and crew. What has this got to do with hydrogen, you may well ask? Well, unstable hydrogen was the gas used to lift this enormous aircraft off the ground. Inert (and non-flammable) Helium was also available but United States law prohibited the export of the gas to Germany (and other countries) since it feared that it would be used for military purposes. Ironically, the worst-ever airship disaster involved the helium-filled USS Akron four years earlier – it crashed in a storm (also in New Jersey).
Compounds
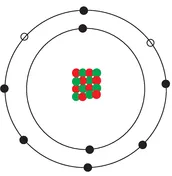
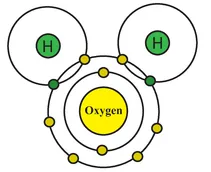
Another extremely unstable element (and highly flammable gas) is oxygen. Yes, good old oxygen is not as well balanced as we give it credit for. Oxygen has eight protons in its nucleus and eight electrons in its two shells (Figure 1.3). This leaves two available spaces that oxygen is restlessly looking to fill. Just like hydrogen then, oxygen suffers from a profound inadequacy complex that often leads to anti-social and volatile behaviour. As mentioned above, oxygen (in sufficient concentration) reacts rather aggressively when exposed to a naked flame. One of the common misconceptions about the earth’s atmosphere is that it is composed entirely of oxygen. If we consider this proposition for a second it doesn’t take long to formulate an experiment to disprove it: if I light a match, will the entire world explode? No. In fact, only 21% of the atmosphere is oxygen (78% is nitrogen) which, fortunately for cigarette smokers and everyone else, is insufficient to pose a threat when lighting a match. However, oxygen is self-evidently a chemically active element and it is not too fussy how it fills its spare electron capacity. Oxygen’s most enduring and lasting relationship (you might even say the love of its life) is with that other highly strung element – hydrogen. Actually, it is more of a ménage à trois since it involves one oxygen atom and two hydrogen atoms. Since hydrogen has one electron and one space in its electron shell, and oxygen has six electrons and two spaces in its outer electron shell, it is possible for oxygen to bond with two hydrogen atoms simultaneously and for all three to achieve a complete outer shell (Figure 1.4). When two different elements combine to form a new molecule it is known as a compound. In this case, the elements hydrogen and oxygen combine to form the compound H2O or water – the most important compound of them all for organic life.
Figure 1.3 Oxygen atom with two empty spaces in outer electron shell.
Figure 1.4 Water (H2O) compound.
Covalent and Hydrogen Bonding
The way in which the two atoms of hydrogen and one atom of oxygen combine is called covalent bonding. The term valence simply refers to the number of electrons in an atom’s...