![]()
CHAPTER ONE
Introduction
Why Scale?
Es gibt überall einen Makroksomos und einen Mikrokosmos, eine Welt im Grossen und eine Welt im Kleinen, diese eben so wichtig, oft wichtiger als jene.1
—Carl Kreil (1865: 2–3)
Scaling is central to the human condition. There are literally dozens of definitions of scale derived from homonyms and cognates rooted in Scandinavian, Germanic, and Romantic languages (OED Online 2018b). These definitions include seven distinct roots and meanings of scale as a noun and three as a verb. All of these variants have subsidiary definitions, ranging from bowls, husks, huts, and balances, through horny coverings of insects, fishes, and plants, to ladders and the scala naturae.
Across science and philosophy, scaling in the sense of inductive reasoning or generalized modeling has an equally long history.2 Levins’ (1966) identification of trade-offs in models between generality, realism, and precision continues to inform and bedevil how we understand the world and forecast changes to it (Weisberg 2013).
Levin (1992: 1943) asserted that “the problem of pattern and scale is the central problem in ecology, population unifying biology and ecosystem science, and marrying basic and applied ecology.” Ecological definitions of scale seem to be derived from the Latin (a ladder or stairway), which entered English usage in the seventeenth or eighteenth century, and most ecological uses of scale reflect a “common-sense” descriptor of spatial (big or small), or temporal (long or short) dimension (Ellison 2018). Despite this common etymology, Levin (1992) argued that there is no single natural scale at which ecological systems or phenomena should be studied because different mechanisms operate at different spatial, temporal, biological, or organizational scales (figure 1.1; cf. figure 2 of Levin 1992). Levin also noted that the spatial or temporal scale of an analysis rarely is motivated by a particular mechanism but more typically reflects an investigator’s perceptual bias or sampling constraints (i.e., the scale at which we choose to observe a system). Other ecologists have turned away from Levin’s scale-specific view of nature, instead searching for scale-free patterns and processes that operate in (nearly) identical ways at all spatial and temporal scales. Ecophysiologists, biomechanists, and macroecologists, among others, have followed the lead of physical scientists who think of scale in terms of dimensionless ratios that characterize processes or mechanisms.
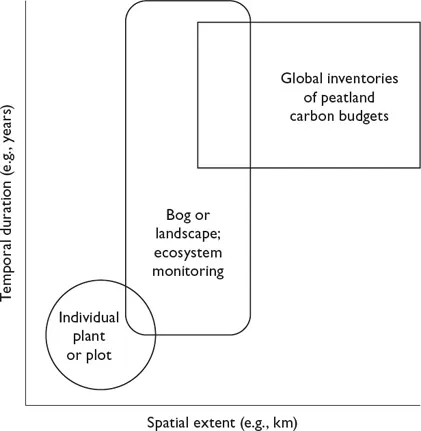
FIGURE 1.1. Different ecological phenomena are measured most commonly at different spatial and temporal scales. Levin’s approach to scaling can be represented on a graph with two axes and a third represented by contours of studies, responses, or levels of inference (Estes et al. 2018).
1.1 TIME AND SPACE
We have adopted these and other approaches to scaling Sarracenia. Like most ecologists, we have implicitly adopted Levin’s approach to scaling. We have studied ecophysiological processes of individual plants measured from seconds (e.g., photosynthesis: Ellison and Gotelli 2002; Ellison and Farnsworth 2005) to seasons (e.g., nutrient uptake, storage, and retranslocation: chapter 3; Ellison and Gotelli 2002; Wakefield et al. 2005; Ellison 2006; Butler and Ellison 2007; Butler et al. 2008; Karagatzides and Ellison 2009; Karagatzides et al. 2009). We have documented and modeled demographic patterns of populations from seasons to decades within and among the bogs where Sarracenia grows (chapter 6; Gotelli and Ellison 2002, 2006b; Ne’eman et al. 2006). We have studied the complex food webs that live within Sarracenia pitchers (chapter 9) at spatial scales ranging from individual pitchers (Gotelli and Ellison 2006a; Butler et al. 2008; Peterson et al. 2008; Gotelli et al. 2011) to the North American continent (Buckley et al. 2003, 2004, 2010; Baiser et al. 2011, 2013), and the temporal scales of their formation (Lau et al. 2018) (figure 1.2). When discussing the “Sarracenia microecosystem” in the remainder of this book, we draw on data collected from within the entire S. purpurea complex (including S. rosea; see appendix A, §A.1.3). We specify geographical, subspecific, or varietal distinctions only when these could help to explain observed patterns.
As we moved our research scale from the lower left to the upper right of figure 1.1, we also traversed the different ecological subdisciplines that typically represent these spatial and temporal scales: physiological and population ecology, community ecology, ecosystem ecology, landscape ecology, macroecology, and biogeography. Each of these subdisciplines has its own research cultures, journals, and meetings, and they interact less often than might be expected. These separate scales of inference and distinct subdisciplines reinforce Levin’s argument that there is no single or natural process, equation(s), or dimensionless number(s) with which to examine ecological phenomena or at which ecological processes operate. However, there might be characteristic equations within each of these scales that could be coupled across scales (§1.3).
1.2 GENES TO ECOSYSTEMS
An alternative interpretation of scale describes the hierarchical levels of biological organization: from genes and genomes to organisms, populations, and ecosystems. This scale has both parallels and some overlap with the time-and-space scales of figure 1.1. The boundaries set by different scales of biological organization also are manifest in the structuring of academic departments and funding agencies. These structural constraints, along with differences in the technical skills, language, and conceptual frameworks (e.g., reductionism, holism, emergence), have made it challenging to explore and transcend boundaries of biological scales. Mechanistic explanations that cross biological scales often move in a linear reductionist chain (e.g., selfish genes drive individual behavior, which in turn affects population-level dynamics). However, our work has helped us to understand patterns and processes that jump scales in complex sequences. For example, nutrient inputs affect pitcher-plant growth (chapter 3), which not only affects demography and population persistence (chapters 6 and 7) but also directly influences food-web development (chapter 9) and alternative ecosystem states (chapters 12 and 13).
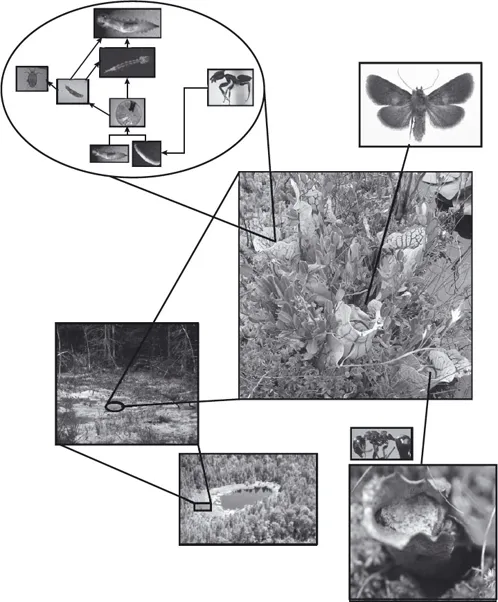
FIGURE 1.2. The Sarracenia purpurea microecosystem includes pitcher plants (center); an aquatic, detritus-based food web within each pitcher (top left); moth larvae that feed on rhizomes, pitchers, and seeds (top right); and ants that nest in old pitchers (bottom right) that are the primary prey of the plant (and the detrital input for the food web). Pitcher plants occur in individual bogs, which are isolated features of larger landscapes (bottom left). Original photographs and photomontage ©Aaron M. Ellison and used with permission.
1.3 MODELING: METABOLIC THEORY AND MACROECOLOGY
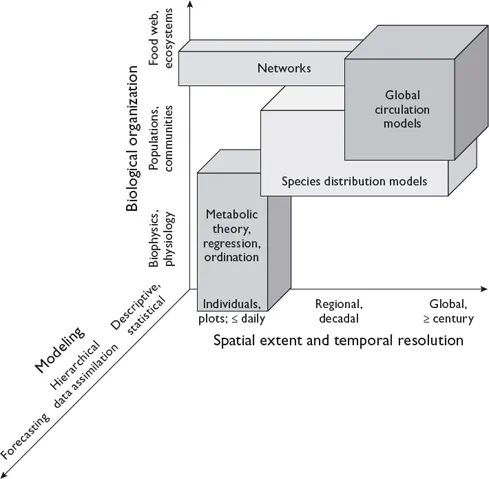
Ecological modeling provides other ways to cross scales (figure 1.3). Although some models capture processes and dynamics of multiple scales of space, time, or biological organization, there is as yet no unified ecological process model that encompasses all of these scales. Metabolic theory and its extensions into macroecology offer an important alternative to scale-specific frameworks and a first step toward a unified model of ecological processes (e.g., West et al. 1997; Enquist et al. 2003; Brown et al. 2004; Shade et al. 2018). The emphasis in macroecology has been on measuring one or more aspects of organismal body size and exploring the functional and mathematical relationships among them. Such dimensional analyses might yield a general process equation for multiple spatiotemporal scales (figure 1.1) or provide links to the processes operating between different scales (figure 1.3).
Although the starting point of West et al. (1997) was the assertion that biological diversity is essentially a reflection of body size (mass), they subsequently used the general allometric (i.e., power law) equation, , to relate body mass (M) to a diversity of physiological and metabolic variables (Y), including metabolic rate, lifespan, cross-sectional area of vessels, and the like. Macroecologists have now documented many examples of allometric scaling of ecological phenomena. Of particular interest are systemic differences between sublinear scaling (b 1 in the power-law equation) and superlinear scaling (b > 1). Phenomena that scale sublinearly show efficiencies of scale, and many biological scaling relationships are sublinear (e.g., the global spectrum of leaf traits, Wright et al. 2004, and see chapter 4). In contrast, phenomena that scale superlinearly are more productive at larger scales. Examples of superlinear scaling relationships are rare—West (2017) found them mostly in cities, companies, and other human organizations—but they may be emergent properties of particular kinds of networks, including ecological ones (Zhang et al. 2015, and see chapters 9 and 10). The “Holy Grail” of metabolic theory and macroecology is the identification of a single underlying mechanism for the wide range of ecological patterns characterized by scaling laws. Common patterns such as quarter-power scaling for physiological processes suggest common mechanisms, but Levin’s observation that “correlations are no substitute for mechanistic understanding” (Levin 1992: 1960) bears repeating.
FIGURE 1.3. Different types of models can cross scales of time, space, and biological organization. This figure was inspired by a 2017 presentation by Scott Doney (University of Virginia) and concepts presented in Gruber and Doney (2009). Redrawn from Ellison (2018) and reproduced under terms of the Creative Commons Attribution License (CC-BY).
1.4 MECHANISMS AT SCALES
By defining function in terms of relationships between levels of organization, Farnsworth et al. (2017) highlighted the role of interactions—and the importance of networks of interactions—as a proximate cause (i.e., a one-level-lower mechanism) of many ecological phenomena. They suggested that experimental manipulations that distinguish effects of network structure (emergent, community-level effects) from effects of traits (organism-level effects) could provide new insights into emergent properties of ecological communities, entire ecosystems, and their relationships (Barry et al. 2019). Our experimental manipulations of the Sarracenia microecosystem (chapters 9 and 12) illustrate the power of this approach.
Our studies of tipping points in the Sarracenia microecosystem (chapters 12 and 13; Sirota et al. 2013; Northrop et al. 2017; Lau et al. 2018) explicitly take advantage of dimensionless (i.e., scale-independent) variables. We have also used comparative studies of carnivorous plants, including species in the genus Sarracenia (Farnsworth and Ellison 2008), to explore taxon-specific divergence from the macroecological scaling laws for leaf traits (chapter 4; West et al. 1997; Wright et al. 2004). The deviations from general scaling exponents detected in these and other counterexamples tend to disappear, or at least regress to the mean, when aggregated data are plotted on a double-log scale.
1.5 ORGANISMS AS MODEL SYSTEMS
A final, perhaps less-intuitive, approach to scaling is the use of model systems in biological research. A handful of species—the bacterium Escherichia coli (Migula), brewer’s yeast Saccharomyces cervisea Meyen ex E. C. Hansen, the cellular slime mold Dictyostelium discoidium Raper, the roundworm Caenorhabditis elegans (Maupas), the fruit fly Drosophila melanogaster Meigen, the house mouse Mus muculus L., and the mouse-eared cress Arabidopsis thaliana (L.) Heynh.—have been developed as “model organisms” by geneticists and molecular, cell, and developmental biologists. These species were not chosen for particular properties. Rather, their utility for answering a broad range of questions that span levels of biological organization emerged over time for a variety of reasons: ease of breeding, rearing, and cultivation in the laboratory; short generation times; and early attention to their underlying genotypes (and later, genomes), genetic mutations, and phenotypic variants that could be maintained in stock cultures. Each of these model organisms also had a “champion,” an individual scientist who, along with their students and colleagues, developed research networks based on the species and encouraged the sharing of cultures, genotypes, phenotypes, and data to address theoretical questions of general interest (Fields and Johnston 2005).
We nominate Sarracenia purpurea as a model ecological species (see appendix A for the fine-grained details of this remarkable plant) but recognize that we are bucking the ecological tradition of eschewing model organisms. Most ecologists are drawn to experimental or observational work in the field, but model organisms have been bred and optimized for work done in laboratories, growth cha...