
eBook - ePub
Adsorption Technology in Water Treatment
Fundamentals, Processes, and Modeling
This is a test
- 376 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Book details
Book preview
Table of contents
Citations
About This Book
This book treats the theoretical fundamentals of adsorption technology for water treatment from a practical perspective. It presents all the basics needed for experimental adsorption studies as well as for process modeling and adsorber design. According to the increasing importance of micropollutants in the water cycle, particular attention is paid to their competitive adsorption in the presence of background organic matter.
The current edition considers recent developments in adsorption theory and practice.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Adsorption Technology in Water Treatment by Eckhard Worch in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Chemistry. We have over one million books available in our catalogue for you to explore.
Information
1 Introduction
1.1 Basic concepts and definitions
1.1.1 Adsorption as a surface process
Adsorption is a phase transfer process that is widely used in practice to remove substances from fluid phases (gases or liquids). It can also be observed as a natural process in different environmental compartments. The most general definition describes adsorption as an enrichment of chemical species from a fluid phase on the surface of a liquid or a solid. In water treatment, adsorption has been proved as an efficient removal process for a multiplicity of solutes. Here, molecules or ions are removed from the aqueous solution by adsorption onto solid surfaces.
Solid surfaces are characterized by active, energy-rich sites that are able to interact with solutes in the adjacent aqueous phase due to their specific electronic and spatial properties. Typically, the active sites have different energies, or – in other words – the surface is energetically heterogeneous.
In adsorption theory, the basic terms shown in Figure 1.1 are used. The solid material that provides the surface for adsorption is referred to as the adsorbent; the species that will be adsorbed are named adsorbate. By changing the properties of the liquid phase (e.g. concentration, temperature, pH) adsorbed species can be released from the surface and transferred back into the liquid phase. This reverse process is referred to as desorption.
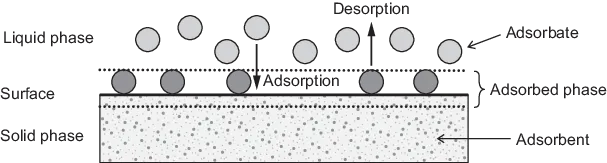
Figure 1.1: Basic terms of adsorption.
Since adsorption is a surface process, the surface area is a key quality parameter of adsorbents. Engineered adsorbents are typically highly porous materials with surface areas in the range between 102 and 103 m2/g. Their porosity allows realizing such large surfaces as internal surfaces constituted by the pore walls. In contrast, the external surface is typically below 1 m2/g and therefore of minor relevance. As an example, the external surface of powdered activated carbon with a particle density of 0.6 g/cm3 and a particle radius of 0.02 mm is calculated to be only 0.25 m2/g, whereas typical values of the internal surface of activated carbons are in the range between 600 and 1,200 m2/g.
1.1.2 Some general thermodynamic considerations
In thermodynamics, the state of a system is described by fundamental equations for the thermodynamic potentials. The Gibbs free energy, G, is one of these thermodynamic potentials. In surface processes, the Gibbs free energy is not only a function of temperature (T), pressure (p), and composition of the system (number of moles, ni) but also a function of the surface area, A. Its change is given by the fundamental equation
(1.1)
where S is the entropy, V is the volume, µ is the chemical potential, and σ is the surface free energy, also referred to as surface tension
(1.2)
If adsorption takes place, the surface free energy is reduced from the initial value σws (surface tension at the water-solid interface) to the value σas (surface tension at the interface between adsorbate solution and solid). The difference between σws and σas depends on the adsorbed amount and is referred to as spreading pressure, π,
(1.3)
The Gibbs fundamental equation (eq. (1.1)) and the relationship between spreading pressure and adsorbent loading provide the basis for the most frequently applied competitive adsorption model, the ideal adsorbed solution theory (Chapter 4).
Conclusions on the heat of adsorption can be drawn by inspecting the change of the free energy of adsorption and its relation to the changes of enthalpy and entropy of adsorption. The general precondition for a spontaneously proceeding reaction is that the change of free energy of reaction has a negative value. Considering the relationship between free energy, enthalpy, and entropy of adsorption, the respective condition for a spontaneous adsorption process reads
(1.4)
The change of the adsorption entropy describes the change in the degree of disorder in the considered system. Typically, the immobilization of the adsorbate leads to a decrease of disorder in the adsorbate/adsorbent system, which means that the change of the entropy is negative (ΔSads < 0). Exceptions could be caused by dissociation during adsorption or by displacement processes where more species are desorbed than adsorbed. Given that ΔSads is negative, it follows from eq. (1.4) that adsorption must be an exothermic process (ΔHads < 0).
Depending on the value of the adsorption enthalpy, adsorption can be categorized as physical adsorption (physisorption) or chemical adsorption (chemisorption). The physical adsorption is caused by van der Waals forces (dipole-dipole interactions, dispersion forces, induction forces), which are relatively weak interactions. The adsorption enthalpy in the case of physisorption is mostly lower than 50 kJ/mol. Chemisorption is based on chemical reactions between the adsorbate and the surface sites, and the interaction energies are therefore in the order of magnitude of reaction enthalpies (>50 kJ/mol). It has to be noted that the differentiation between physisorption and chemisorption is widely arbitrary and the boundaries are fluid.
1.1.3 Adsorption versus absorption
As explained before, the term “adsorption” describes the enrichment of adsorbates on the surface of an adsorbent. In contrast, absorption is defined as transfer of a substance from one bulk phase to another bulk phase. Here, the substance is enriched within the receiving phase and not only on its surface. The dissolution of gases in liquids is a typical example of absorption.
In natural systems, some materials with complex structure can bind substances from the aqueous phase on their surface but also in the interior of the material. The uptake of organic solutes by the organic fractions of soils, sediments, or aquifer materials is a typical example of such complex binding mechanisms. In such cases, it is not easy to distinguish between adsorption and absorption. Therefore, the more general term “sorption” is preferred to describe the phase transfer between the liquid and the solid in natural systems. The term “sorption” comprises adsorption and absorption. Moreover, the general term “sorption” is also used for ion exchange processes on mineral surfaces.
1.1.4 Mathematical description of adsorption processes: The structure of the adsorption theory
In accordance with the character of adsorption as a surface process, it would be reasonable to express the adsorbate uptake by the adsorbent surface as surface concentration, Γ (in mol/m2), which is the quotient of the adsorbed amount, na, and the adsorbent surface area, A,
(1.5)
However, since the surface area, A, cannot be determined as exactly as the adsorbent mass, in practice the mass-related adsorbed amount, q, is typically used instead of the surface concentration, Γ,
(1.6)
where mA is the adsorbent mass. The amount adsorbed per mass adsorbent is also referred to as adsorbent loading or simply loading.
In view o...
Table of contents
- Title Page
- Copyright
- Contents
- 1 Introduction
- 2 Adsorbents and adsorbent characterization
- 3 Adsorption equilibrium I: General aspects and single-solute adsorption
- 4 Adsorption equilibrium II: Multisolute adsorption
- 5 Adsorption kinetics
- 6 Adsorption dynamics in fixed-bed adsorbers
- 7 Fixed-bed adsorber design
- 8 Desorption and reactivation
- 9 Geosorption processes in water treatment
- 10 Appendix
- Nomenclature
- Index