
eBook - ePub
Chiral Ligands
Evolution of Ligand Libraries for Asymmetric Catalysis
This is a test
- 322 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Chiral Ligands
Evolution of Ligand Libraries for Asymmetric Catalysis
Book details
Book preview
Table of contents
Citations
About This Book
Many new drugs on the market are chiral compounds, that is, they can exist in two non-superimposable mirror-image forms. Asymmetric catalysis encompasses a large variety of processes for obtaining such compounds. The performance of the catalyst in those processes largely depends on the ligand that makes up the catalyst. This book describes the most relevant ligand libraries for some key processes, including an overview of the state of art and the key mechanistic aspects that favor a high catalytic performance.
Key Features:
- The book presents historical content from the time of discovery for each family of ligands.
- Provides a description of the synthetic route and the ligand library's application in various catalytic asymmetric reactions
- Suitable as supplementary reading for courses targeting the design, synthesis and application of chiral catalysts, asymmetric catalysis and sustainable production
- Edited by a distinguished scientist in the field, the book has a diverse audience including research groups in homogeneous catalysis, particularly asymmetric transformations
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Chiral Ligands by Montserrat Diéguez, Montserrat Diéguez in PDF and/or ePUB format, as well as other popular books in Medicine & Biochemistry in Medicine. We have over one million books available in our catalogue for you to explore.
Information
1 Chiral Bidentate Heterodonor P-Oxazoline Ligands
Maria Biosca , Jordi Faiges , Montserrat Diéguez , and Oscar Pàmies
Contents
1.1 Introduction
1.2 Application of Bidentate Heterodonor P-Oxazoline Ligands in Asymmetric Catalytic Transformations
1.3 Conclusions
1.4 Acknowledgements
References
1.1 Introduction
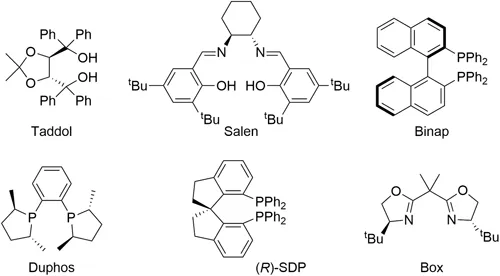
The performance of enantioselective metal catalysts depends mainly on the selection of the most appropriate chiral ligand.1 Among the thousands of chiral ligands which have been developed, a few stand out for their broad applicability. The most efficient, called "privileged chiral ligands", derive from a few core structures.2 Surprisingly, most of them possess C 2 symmetry (Figure 1.1). The reason for initially choosing bidentate ligands with C 2-symmetry was to reduce the number of catalyst/substrate arrangements and transition states, facilitating mechanistic studies, and the elucidation of the relationship between structure and catalytic performance. So, for a long time, the research focused on developing C 2-symmetric ligands. However, the intermediate transition metal ligand complex that arises during a catalytic cycle may not be symmetric and, in these cases, the desymmetrization of the ligand, by tuning each donor atom to accommodate a specific purpose in the catalytic cycle, has been shown to achieve better enantiocontrol for some reactions. One of the most effective methods of desymmetrizing a ligand is by using different donor atoms. In the last few decades, heterodonor ligands, containing dual, strongly and weakly donor heteroatom pairs, have emerged as an increasingly useful ligand class, since the different electronic and steric properties of these heteroatoms are powerful stereocontrol elements.3 The two functionalities also facilitate catalyst optimization, because both functionalities can be independently modified for improved performance. Among them, P–N ligands have been the most-widely-used P–oxazolines, as a result of being the most- studied combination, due to their ready accessibility and modular construction. 3a-e The vast majority of P-oxazoline ligands are derived from readily available chiral amino alcohols, in short and efficient synthetic sequences. 3a-e
1.2 Application of Bidentate Heterodonor P-Oxazoline Ligands in Asymmetric Catalytic Transformations
The origin of P-oxazoline ligands can be traced back to 1993, with three independent publications from Helmchem, Pfaltz, and Williams, with the introduction of a new versatile class of ligands, the phosphine-oxazoline PHOX ligands 1 (Figure 1.2).4 PHOX ligands have been successfully applied to many metal-catalyzed asymmetric reactions, such as hydrogenation, inter- and intramolecular Heck reactions, allylic substitutions and decarboxylative allylation reactions, conjugate additions to enones, Diels-Alder and aza-Diels-Alder reactions, among others.2 Due to this extensive success, PHOX is the only heterodonor ligand included in the family of “privileged ligands”.2 Despite having been described more than 25 years ago, they continue to be used in new asymmetric transformations, underlining their status as a privileged chiral ligand class.5
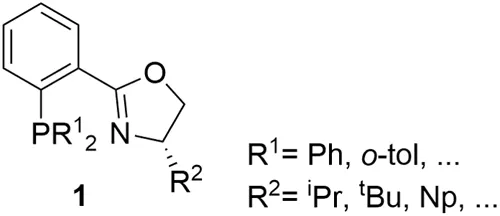
Inspired by the PHOX ligands, several variations on P-oxazoline ligands have been made by changing either the ligand scaffold or the properties of the phosphine group, or by substituing the phosphine moiety by other P-donor groups.3a-e These specific modifications of the simple PHOX ligands achieved improvements in enantioselectivity in some cases. However, only a few of them have been successfully applied to several, mechanistically unrelated, asymmetric reactions, with a broad substrate/reagent range. A broad range of reactions, and a wide substrate/reagent scope are desirable to minimize the time necessary to achieve ligand discovery and preparation. Figure 1.3 presents a selection of the most representative families of P-oxazoline ligands, developed for use in metal-catalyzed asymmetric transformations.

Some of these successful modifications are electronic, by introducing electron-withdrawing groups in the phenyl backbone ring and/or in the phosphine moiety (e.g., ligands L1). Another modification includes the replacement of the phosphine group by biaryl phosphite groups, modifying the electronic and steric properties of the ligand (ligands L2). Other modifications are on the oxazoline group, by attaching the phenyl backbone ring to the stereogenic center next to the oxazoline (ligands L3), or introducing other oxazoline substituents, such as ferrocene, tricyclic, and sugar oxazoline groups (e.g., ligands L4–L5). Another modification of the oxazoline ring was to introduce substituents in the 5 and/or 5' positions (e.g., ligands L6 and L7), providing levels of enantio-induction similar to that of the usually most effective PHOX ligand, the tBuPHOX, but with the advantage of being readily accessible as both enantiomers from either the (S)- or (R)-valine rather than from expensive tert-leucinol enantiomers. Some of these modifications on the phosphine and oxazoline moieties, in the phenyl backbone ring, and many changes on the ligand backbone have been studied. Examples include inclusion of a methylene spacer between the oxazoline ring and the phenyl ring on the ligand backbone (ligands L8), and the replacement of the phosphine group of ligands L8 by biaryl phosphite moieties (ligands L9). Many of the backbone changes includes the replacement of the phenyl backbone ring of the PHOX ligand by other moieties, such as ferro- and ruthenocene groups (e.g., ligands L10–L14), biphenyl or binapthyl groups (e.g., ligands L15), several heterocyclic backbones (e. g., ligands L16–L19), an alkyl chain (e.g., ligands L20–L28) and byciclic, sugar, and spiro backbones (e.g., ligands L29–L34). In many of these latter backbone modifications, the phosphine group has also been replaced by a phosphinite, phosphite, aminophosphine, or even stereogenic P groups.
In next sections, we collect the catalytic results on asymmetric catalysis from those new P-oxazoline ligand libraries, that have been successfully applied in a broad reaction/substrate/reagent scope and the relationship between their architectural design and their catalytic performance. We will focus on recent reports, and a short overview of previous work in the field will also be included.
A notable modification of the electronic properties of PHOX ligands was the development of ligands L1 by Stoltz’s group (R2= tBu). In 2007, they discovered that the use of L1 was highly beneficial in the decarboxylative allylation of cyclic allyl carbonates, providing greater asymmetric induction and activities than traditional PHOX ligands (Figure 1.4a).6 This early finding paved the way to the total synthesis of a series of natural products, ...
Table of contents
- Cover
- Half-Title
- Series
- Title
- Copyright
- Contents
- Preface
- Editor
- List of Contributors
- Abbreviations and Acronyms
- Chapter 1 Chiral Bidentate Heterodonor P-Oxazoline Ligands
- Chapter 2 Chiral Bidentate Heterodonor P-N-Other-Ligands
- Chapter 3 Chiral Bidentate Heterodonor P,S/O Ligands
- Chapter 4 Chiral Bidentate Heterodonor P-P’ ligands
- Chapter 5 Chiral Tridentate-Based Ligands
- Chapter 6 Chiral N-Heterocyclic Carbene-Based Ligands
- Chapter 7 Chiral Monophosphorus Ligands
- Chapter 8 Solvent-Oriented Ligand and Catalyst Design in Asymmetric Catalysis – Principles and Limits
- Index