
- 126 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Green Extraction in Separation Technology
About this book
Subcritical water is a green extraction solvent compared to conventional extraction solvents. While experimental results on subcritical water extraction (SWE) technology have been published piecemeal, there has been no comprehensive review of the state of the art. Green Extraction in Separation Technology fills that gap, serving to cover extracting with subcritical water as an environmentally friendly solvent.
FEATURES
- Presents new technologies for extracting natural compounds from plants and compares the advantages and disadvantages versus SWE
- Explains research on SWE over the last 15 years
- Offers an overview of the solubility of different compounds in SWE and related theoretical content
- Discusses modeling of SWE and describes the development of a new model for this process
This monograph is aimed at researchers and advanced students in chemical and biochemical engineering.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1
New Technologies for Extracting Natural Compounds from Plants
1.1 ESSENTIAL OILS
Essential oils or essences are aromatic compounds that are widely used in the perfume, pharmaceutical and food industries. Essential oils are a mixture of more than 200 compounds that can be divided into two general categories. One part is volatile compounds that make up about 90-95% of total oil and contain monoterpene and sesquiterpene hydrocarbons and their oxygen derivatives along with aldehydes, alcohols, aliphatic esters, ketones, lactones and phenols. The other part is non-volatile residue that makes up 5–10% of the oil and contains hydrocarbons, fatty acids, sterols, carotenoids, waxes and flavonoids [1, 2].
Figure 1.1 schematically shows the heterogeneous chemical groups in the essential oils. The terpene component of essential oils has very little effect on the aroma of essential oils, and since terpenes are mainly unsaturated compounds, they decompose by heat, light and oxygen to produce unwanted compounds with an unpleasant odor. The oxygen-containing part of the essential oil is very fragrant, and mainly the main specifications of the essential oil are related to this part. It has oxygen compounds.
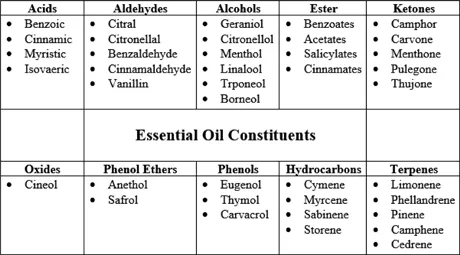
Figure 1.1 Heterogeneous chemical groups present in essential oil.
Essential oils are classified into three groups: (1) Natural essential oils are products obtained from plant raw materials by one of the extraction methods (distillation, mechanical pressing and extraction with solvent), (2) semi-natural essential oils are products that are formed from a combination of aromatic raw materials and are similar in smell to natural essential oils, (3) artificial essential oils are products that are commercially produced from organic chemicals similar to natural essential oils and have an odor similar to natural essential oils.
In general, each method of separation from solid matrix consists of two steps: Extraction (such as single-stage solvent extraction, Soxhlet extraction, steam distillation, simultaneous distillation extraction, etc.) and analysis (gas chromatography and gravimetry, etc.). While the analysis step is complete after only 2–20 min, extraction takes at least a few hours. Separation from the solid matrix is often performed by prolonged heating and stirring in the solvent. Therefore, in principle, extraction is the main limiting step, which includes the transfer of desirable compounds in the solvent. The usual solvent extraction method involves 70% of the total separation process time. Therefore, it is very important to shorten this restrictive step. The choice of a method is the result of an agreement between yield, repeatability of the extraction, ease of the procedure, taking into account the cost, time of the process, the degree of automation of the process and safety.
Traditionally, solid matter extraction is performed by the Soxhlet extraction method. This method is obtained by the interaction of the sample with solvent condensed vapors. Soxhlet has been one of the most widely used methods of solid-liquid extraction for a long time and is currently the main reference method. Soxhlet extraction from solids has undeniable advantages such as continuous extraction with repeated infiltration of fresh solvent, no need for treatment stage and the possibility of solvent recovery. However, this method has some disadvantages, including poor extraction of polar compounds, long operating time, large solvent volume, solvent boiling point operation and is unsuitable for unstable thermal analyzes.
These shortcomings have led to the use of new “fast” and “green” techniques in sample preparation, which typically use less solvent and less energy, such as ultrasound-assisted extraction (UAE), supercritical fluid extraction (SFE), microwave extraction, controlled pressure drop process, accelerated solvent extraction (ASE) and subcritical water extraction (SWE).
At present, the extraction and preparation of samples under conditional or non-classical conditions is a dynamic developing area in analytical chemistry. Using these “quick” and “green” sample preparation methods, extraction and distillation can now be performed in a matter of minutes instead of hours with high repeatability, reduced solvent consumption and simple manufacturing, achieving higher purity of the final product, eliminating pre-treatment waste and consuming only a fraction of the energy required against conventional sampling methods, such as Soxhlet extraction, Clevenger, Dean-Stark method.
This chapter provides a brief overview of current knowledge of innovative methods of extracting natural compounds from plants. This study provides the necessary theoretical background and some details about extraction using innovative, fast and green techniques such as ultrasound, microwave, controlled pressure drop process, supercritical fluid extraction, subcritical water extraction, the method of performance, application and their environmental effects.
1.2 DEFINITION OF EXTRACTION
Extraction is one of the most important processes in separation. Extraction of a part of a solid matrix can be considered as a five-step process: Attracting a combination of active sites of the matrix, its diffusion into the matrix; dissolved in extract, diffusion of composition into the extract and collection of extracted components (Figure 1.2). Optimal optimization and control of each stage, especially the extract collection stage, is necessary. In environmental applications (for example, the extraction of pollutants from soils and sediments), the first step is usually to limit the speed, because the effects of the extraction material matrix are very difficult to overcome and predict. For other matrices (for example, plant material), speed may be controlled by solubility or penetration. Therefore, optimization strategies depend on the nature of the matrix.
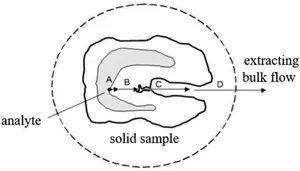
Figure 1.2 Steps of extraction from solid samples.
1.3 DEFINITION OF GREEN EXTRACTION
The general definition of green chemistry is the invention, design and application of chemical products and processes to reduce or eliminate the use and production of hazardous materials. Regarding the green extraction of compounds from plants, this definition has been amended as follows: “Green extraction is based on recognizing and designing extraction processes that reduce energy consumption, increase the use of alternative solvents, and ensure the safety of a high-quality product or extract” [3].
1.4 DISTILLATION WITH WATER OR STEAM
One of the most common methods of extracting essential oils is distillation with water or steam. Based on the water or steam distillation method, three systems of water distillation, water-steam distillation and steam distillation have been designed and built so far such that steam distillation system is generally used on an industrial scale. In this way, water vapor is placed at low pressure (1.0 bar) and the steam, after passing through the plant mass and extracting the compounds, collects its essential oil in the cooling part of the system.
Disadvantages of this method are the risk of loss of volatile compounds against heat, the impracticality of the process automatically and the long time for extraction. However, in order to prevent the loss of volatile compounds against heat, vacuum distillation is used, which is the process of distillation of water vapor that is performed at low pressure. However, this method still has two significant disadvantages mentioned above.
1.5 NEW TECHNOLOGIES FOR EXTRACTING NATURAL COMPOUNDS
As mentioned, traditional methods have given way to new methods due to their time-consuming extraction and high solvent consumption. Therefore, there is a great demand for new extraction methods with shorter time, lower solvent consumption and environmental protection. New methods of extracting essential oils such as extraction with ultrasound, extraction with microwave, extraction with supercritical fluid and extraction with subcritical water are very fast and effective for extracting plant compounds.
1.5.1 Microwave-Assisted Solvent Extraction (MASE)
The use of microwave energy was first reported in 1986 simultaneously by Gedbye in organic synthesis [4] and by Ganzler for the extraction of biological samples and analysis of organic compounds [5]. Since then, several laboratories have studied the combined and analytical capabilities of the microwave as a non-classical source of energy. Several categories of compounds such as essential oils, fragrances, pigments, antioxidants and other organic compounds have been effectively extracted from a variety of matrices (mainly animal, food and plant tissues). Advances in microwave extraction have provided two categories of solutions: Microwave-assisted solvent extraction (MASE) and solvent-free microwave extraction (SFME).
MASE was first used to extract several compounds (citrus, aromatic plants, cereals, etc.). Many categories of compounds such as perfumes, antioxidants, dyes, bio phenols and other primary and secondary metabolites have been effe...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Contents
- Preface
- Authors
- 1 New Technologies for Extracting Natural Compounds from Plants
- 2 Review of Subcritical Water Extraction (SWE)
- 3 Solubility of Subcritical Water
- 4 Modeling of Subcritical Water Extraction
- Subject Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Green Extraction in Separation Technology by Ali Haghighi Asl,Maryam Khajenoori in PDF and/or ePUB format, as well as other popular books in Biological Sciences & Biotechnology. We have over one million books available in our catalogue for you to explore.