eBook - ePub
Evolutionary Origin of Sensory and Neurosecretory Cell Types
Vertebrate Cranial Placodes, volume 2
This is a test
- 300 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Evolutionary Origin of Sensory and Neurosecretory Cell Types
Vertebrate Cranial Placodes, volume 2
Book details
Book preview
Table of contents
Citations
About This Book
Most vertebrate cranial sense organs arise from placodes. These placodes give rise to sensory neurons that transmit information to the brain and neurosecretory cells. This book reviews the evolutionary origin of the sensory and neurosecretory cell types. It summarizes our current understanding of vertebrate evolution, clarifies conceptual issues relating to homology and evolutionary innovation of cell types, compares the sensory and neurosecretory cell types with similar cell types in other animals, and addresses the question of how cranial placodes evolved as novel structures in vertebrates by redeploying pre-existing and sometimes evolutionarily ancient cell types.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Evolutionary Origin of Sensory and Neurosecretory Cell Types by Gerhard Schlosser in PDF and/or ePUB format, as well as other popular books in Biological Sciences & Biology. We have over one million books available in our catalogue for you to explore.
Information
1
The Evolutionary Origin of Vertebrates
Within the large branch of animals known as deuterostomes, only vertebrates possess a complex head equipped with complex paired sense organs such as the eyes, the ears (visible from the outside only in some species by the presence of openings, eardrums, or pinnae), the lateral line system (in fishes and amphibians), and the nose. This is immediately obvious in a comparison of vertebrates (Fig. 1.1) with other deuterostomes (Fig. 1.2). Beneath the surface, these profound differences continue with only vertebrates possessing a cartilaginous or bony skull protecting an enlarged brain and a series of cranial nerves which connect the sensory organs to the brain and the brain to the cranial muscles and glands. Some of these muscles help to draw water and food particles through the mouth into the pharynx and then expel it through perforations in the wall of the pharynx, the pharyngeal slits, to the outside. Comparable complex sense organs, a skull and the muscular ventilation of the pharynx are absent from other deuterostomes indicating that they are evolutionary novelties that arose in the vertebrate lineage. In that sense, vertebrates were proposed to have a “New Head” by Northcutt and Gans (Northcutt and Gans 1983; Gans and Northcutt 1983).

FIGURE 1.1 (A) Hagfish (Myxine sp.). (B) Sea lamprey (Petromyzon marinus). (C) Blacktip reef shark (Carcharhinus melanopterus). (D) Short-nose Sturgeon (Acipenser brevirostrum). (E) Arowana (Scleropages legendrei). (F) White margined unicornfish (Naso annulatus). (G) Broadbarred firefish (Pterois antennata). (H) Fire salamander (Salamandra salamandra). (I) Frog (Rana sp.). (J) Large Scaled Forest Lizard (Calotes grandisqamis). (K) Blue-legged Chameleon (Calumma crypticum). (L) Albino Burmese python (Python bivittatus). (M) Red-tailed green rat snake (Gonyosoma oxycephalum). (N) Greylag goose (Ansera anser). (O) Bald eagle (Haliaeetus leucocephalus). (P) Male mongoose lemur (Eulemur mongoz). (Q) Dermanura sp. (R) Jaguar (Panthera onca). (Photos by [A] NOAA Okeanos Explorer Program; [B] P. Lameiro.; [C] Luc Viatour; [D] US Fish and Wildlife service; [E] Marcel Burkhard (CC BY-SA 3.0); [F] Bernard Spragg; [G] H. Zell; [H] Didier Descouens; [I] Luk; [J] Babujayan; [K] Frank Vassen (cc-by-2.0); [L] Guy Lejeune; [M] Bernard Dupont; [N] Charlesjsharp; [O] US Fish and Wildlife Service/Mike Lockhart; [P] IParjan; [Q] Guilherme Garbino; [R] Cburnett.)

FIGURE 1.2 A collection of non-vertebrate deuterostomes. A head with complex sense organs is lacking. (A) Sea star (Asterias rubens). (B) Saccoglossus kowalevskii. (C) Ciona savignyi. (D) Amphioxus (Branchiostoma floridae). ([A] Photo by Hans Hillewaert; [B] reprinted with permission from Gerhart, Lowe, and Kirschner 2005; [C] photo by Steve Lonhart/NOAA MBNMS; [D] Photo kindly provided by David Jandzik.)
In their “New Head” hypothesis, Northcutt and Gans suggested that the New Head evolved in a series of steps during which filter-feeding ancestors adopted a more active and predatory life-style. Filter-feeding is indeed the predominant feeding mode of other chordates and deuterostomes suggesting that it was the feeding mode of the ancestral chordates (Fig. 1.3). Most deuterostomes use mucus to capture small food particles which are then transported by cilia into the pharynx and onwards into the digestive tract. In chordates, most of this mucus is produced in a special ciliated groove in the ventral pharynx termed the endostyle. The pharynx of tunicates, amphioxus as well as hemichordates, and some extinct groups of echinoderms, is perforated by pharyngeal slits to provide an exit route for the water drawn in during filter feeding. The invention of muscular ventilation of the pharynx allowed to generate larger forces to suck in larger food particles precipitating the transition to a more active and exploratory behavior which in turn favored the evolution of increasing sensory capacities and skeletal protection of sense organs and brain.
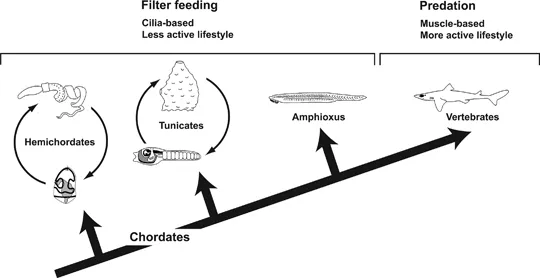
FIGURE 1.3 Origin of vertebrates from suspension feeding chordates. Larval and adult stages are shown for hemichordates and tunicates. See text for detailed explanation. (Silhouettes reprinted with permission from Northcutt 2005.)
Northcutt and Gans further pointed out that many of the evolutionary novelties of the vertebrate head develop from only two embryonic tissues, the neural crest and the cranial placodes (Fig. 1.4) which also originated first in the vertebrate lineage (Northcutt and Gans 1983). The neural crest contributes to the development of the skull and to glial cells and sensory neurons of the cranial nerves, while cranial placodes also contribute sensory neurons to the cranial nerves and in addition give rise to many cranial sense organs and to the neurosecretory cells of the anterior pituitary gland.
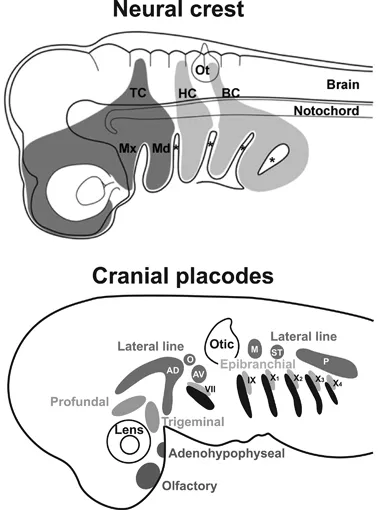
FIGURE 1.4 Neural crest and cranial placodes. Upper panel shows the cranial neural crest streams in a generalized vertebrate embryo. Asterisks mark ectodermal pharyngeal grooves, which will fuse with the underlying pharyngeal pouches to form the pharyngeal slits. BC, branchial crest; HC, hyoid crest; Md, mandible (lower jaw); Mx, maxilla (upper jaw); Ot, otic vesicle; TC, trigeminal crest. Lower panel shows cranial placodes in a generalized vertebrate embryo. These include the adenohypophyseal, olfactory, lens and otic placode, the profundal and trigeminal placodes, and several epibranchial and lateral line placodes. Epibranchial placodes are closely associated with the pharyngeal pouches (shown in black). AD, anterodorsal lateral line placode; AV, anteroventral lateral line placode; O, otic lateral line placode; M, middle lateral line placode; P, posterior lateral line placode; ST, supratemporal lateral line placode; VII, epibranchial placode of facial nerve; IX, epibranchial placode of glossopharyngeal nerve; X1-4, epibranchial placodes of vagal nerve. (Upper panel: Reprinted with permission from Kuratani et al. 2012; Lower panel: Redrawn and modified from Northcutt 1997.)
In the first volume (Schlosser 2021), I introduced the specialized sensory (organs olfactory, ear, lateral line), neurosecretory organs (anterior pituitary) and cranial ganglia of the vertebrate head that arise from cranial placodes. I then reviewed how sensory and neurosecretory cell types arise from cranial placodes during vertebrate embryonic development. I also briefly sketched the development of photoreceptors, even though these do not arise from cranial placodes, because I hope to show that they are evolutionarily related to other, placode-derived sensory cells. In the second volume, I will now attempt to trace the evolutionary history of cranial placodes and their derivative cell types. In this first chapter, I will place vertebrates into their proper phylogenetic context. In Chapter 2, I will then make some general remarks on cell types, and discuss how we can recognize homology and novelty in cell type evolution. In Chapters 3–5, I compare the sensory and neurosecretory cell types of the vertebrate head with similar cell types in other animals to get insights into their evolutionary origins. In the final chapter (Chapter 6), I will then discuss how cranial placodes evolved as novel structures in vertebrates by redeploying pre-existing and sometimes evolutionarily ancient cell types.
To understand how the vertebrate head originated during evolution and how its complex sense organs evolved, we first need to know more about the pedigree of the vertebrates. What did their ancestors look like? How did they develop and live and how did their development and lifestyle change over time? Answering these questions is by no means trivial. Obviously, we have no time machine that would allow us to travel back in time to study the extinct ancestors of the vertebrates living today. And even if this were possible, we still wouldn’t know where to position them on the tree of life. We, thus, must try to reconstruct the evolutionary history and infer the bauplan and life history of ancestors by comparisons with other animals living today or with the fossil remnants of animals of the past. In the remainder of this chapter, I will first summarize our current understanding of the phylogenetic relationships of vertebrates with other deuterostomes and discuss how deuterostomes are phylogenetically related to other metazoans. I will then introduce the vertebrates and their fossil relatives and present a brief survey of other deuterostome groups as the closest living relatives of vertebrates. Finally, I will review different scenarios on how vertebrates and other deuterostomes evolved from their common ancestors.
1.1 VERTEBRATES AND THE TREE OF LIFE
The phylogenetic relationships between vertebrates and other chordates and between chordates and other animals has been controversial for a long time. While not all of these controversies are resolved yet, modern phylogenetic methods provide an increasingly well supported picture of chordate phylogenetic relationships.
1.1.1 A Brief Primer on Phylogenetic Systematics
The methodological principles underlying these phylogenetic methods were first formulated by Willi Hennig in his influential book on phylogenetic systematics (Hennig 1966). A basic assumption of this methodology is that the diversification of taxa (species or other units of classification; singular: taxon) in evolution occurs typically by dichotomous branching, each branching point giving rise to two side branches termed sister groups. This is represented in a branching diagram called a cladogram. Phylogenetic systematics aims to classify taxa in a way that reflects this branching pattern by constructing a hierarchy of monophyletic groups. A monophyletic group (or clade) contains all the side branches emanating from a branching point, i.e. all descendants of a common ancestor (Fig. 1.5A). Birds, for example, are a monophyletic group. All birds arose from a last common ancestor that only gave rise to birds. In contrast, a polyphyletic group would unite two branches arising from different ancestors (such as birds and bats). Finally, a paraphyletic group contains some but not all descendants of a common ancestor. “Reptiles”, for example, are a paraphyletic group because not only “reptiles” but also birds and mammals descended from the last common ancestor of “reptiles”. Only monophyletic groups but not poly- or paraphyletic groups are acceptable in phylogenetic systematics; therefore, quotation marks are often used when referring to groups that are paraphyletic such as “reptiles”.
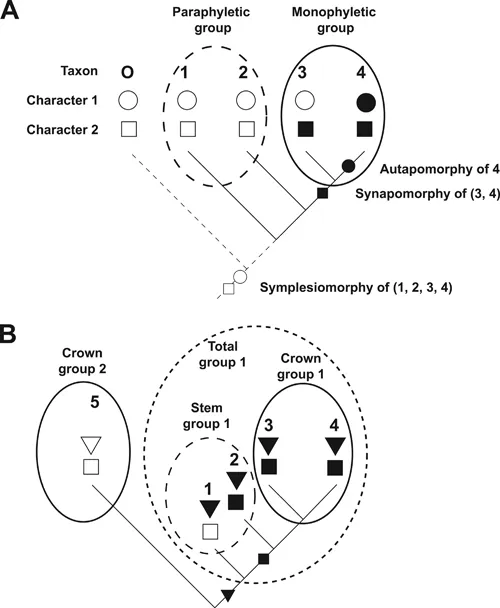
FIGURE 1.5 Terminology of phylogenetic systematic. (A) Cladogram depicting the phylogenetic relationships for four taxa (1–4) and an outgroup (O). Different character states for two characters (circle, square) are indicated by empty or filled symbols. (B) Illustration of the concepts of crown group, stem group, and total group. Taxa 1 and 2 are extinct; taxa 3–5 are extant. Distribution and inferred origin of character states for the various taxa are indicated. See t...
Table of contents
- Cover
- Half Title
- Series Page
- Title Page
- Copyright Page
- Contents
- Preface
- 1 The Evolutionary Origin of Vertebrates
- 2 Teaching Old Cells New Tricks
- 3 Evolution of Mechano- and Chemosensory Cell Types
- 4 Evolution of Photosensory Cell Types
- 5 Evolution of Neurosecretory Cell Types
- 6 Evolutionary Origin of Vertebrate Cranial Placodes
- References
- Index