
Catalysis for Fine Chemicals
- 403 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Catalysis for Fine Chemicals
About this book
A wide range of chemical products (especially fine chemicals) are important for a healthy and enjoyable modern life; therefore efficient syntheses of these materials are essential. Traditional stoichiometric processes need to be replaced by modern catalytical methods in the change to sustainable chemistry and the production of lower amounts of waste.
This book summarizes the wide variety of catalytic methods that have been developed and applied on an industrial scale in recent years to fulfill this goal. The synthesis of compound classes such as pharmaceuticals, agrochemicals, flavoring, and fragrance compounds as well as food additives such as vitamins exemplify the use of these modern catalytic methods in the modern chemical industry.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1 Introduction and fundamental aspects
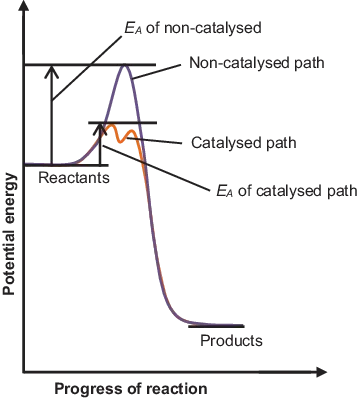
- Mild reaction conditions,
- Decreased waste formation,
- Increased selectivity,
- Increased efficiency.
Table of contents
- Title Page
- Copyright
- Contents
- Preface
- 1 Introduction and fundamental aspects
- 2 Heterogeneous hydrogenations
- 3 Homogeneous hydrogenations
- 4 Oxidations
- 5 Gas-phase reactions
- 6 C–C-bond and C–N-bond forming reactions (metal-catalysed)
- 7 Rearrangement reactions
- 8 Acid–base-catalysed reactions
- 9 Phase transfer catalysis (PTC)
- 10 Biocatalysis
- 11 New trends
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app