
- 258 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Interfacial Chemistry of Rocks and Soils
About This Book
Knowledge of the basic interactions that take place between geological materials and different substances is the first step in understanding the effects of adsorption and other interfacial processes on the quality of rocks and soils, and on driving these processes towards a beneficial or neutral result. Interfacial Chemistry of Rocks and Soils examines the different processes at solid and liquid interfaces of soil and rock, presenting a complete analysis that emphasizes the importance of chemical species on these interactions.
This Second Edition features novel results in the field and expanded coverage of the kinetics of interfacial processes. New content includes models of heterogeneous isotope exchange, sorption isotherms for heterovalent cation exchange, as well as sorption of anions by chemically modified clays.
Summarizing the results and knowledge of the authors' research in this field over several decades, this volume:
-
- Explores the individual components of the studied systems: the solid, the solution, and the interface
-
- Discusses the characteristics and thermodynamics of the interface
-
- Profiles the most important analytical methods in the study of interfacial processes
-
- Demonstrates transformations initiated by interfacial processes
-
- Outlines avenues of treatment that may solve geological, soil science, and environmental problems
Drawn chiefly from the authors' years of research at the Imre Lajos Isotope Laboratory in the Department of Physical Chemistry at the University of Debrecen in Hungary, this book discusses chemical reactions on the surfaces/interfaces of soils and rocks; examines the role of these processes in environmental, colloid and geochemistry; and explores the effects on agricultural, environmental and industrial applications.
Frequently asked questions
Information
1 Components of Soil- and Rock-Solution Systems
1.1 Solid: Soil and Rock
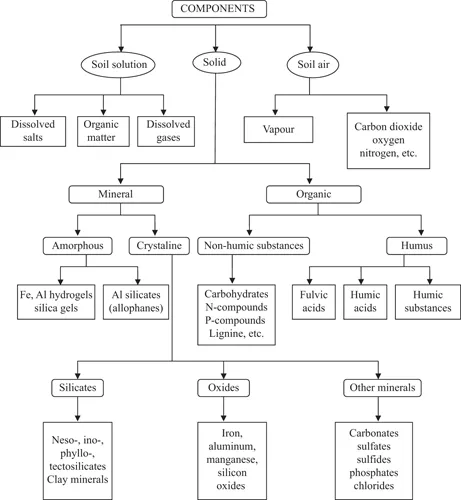
1.1.1 Mineral and Chemical Composition of Rocks and Soils
- Elements.
- Sulfides.
- Halogenides.
- Oxides and hydroxides.
- Nitrates, carbonates, and borates.
- Sulfates, chromates, molybdates, and tungstates.
- Phosphates, arsenates, and vanadates.
- Silicates.
- Organic compounds.
Table of contents
- Cover
- Half-Title
- Series
- Title
- Copyright
- Contents
- Preface to the First Edition
- Preface to the Second Edition
- Authors
- Chapter 1 Components of Soil- and Rock-Solution Systems
- Chapter 2 Interfacial Processes in Geological Systems: Studies on Montmorillonite Model Substance
- Chapter 3 Interfacial Reactions at Rock and Soil Interfaces
- Chapter 4 Experimental Methods in Studying Interfacial Processes of Rocks and Soils
- Index