eBook - ePub
Catheter Ablation of Cardiac Arrhythmias in Children and Patients with Congenital Heart Disease
This is a test
- 330 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Catheter Ablation of Cardiac Arrhythmias in Children and Patients with Congenital Heart Disease
Book details
Book preview
Table of contents
Citations
About This Book
This authoritative book explores electrophysiologic testing and therapeutic catheter ablation for cardiac arrhythmias in children, and in patients of all ages with congenital heart disease. It reviews the anatomic and physiologic background to these procedures, emphasizing the tools for mapping and tissue ablation that continue to improve patient outcomes. Additionally, individual chapters are dedicated to specific congenital heart defects (for instance, tetralogy of Fallot, Ebstein's anomaly, univentricular heart) guiding the reader to anticipate the type of arrhythmia, the most likely location for effective ablation, and the technical challenges that may be encountered in each condition.
Key Features
- Provides a detailed review of the unique challenges presented by young patients with small heart size, and patients of any age with distorted anatomy due to congenital heart disease, in this long overdue, updated text
-
- Intends to guide all cardiologists engaged in invasive electrophysiology at both the training level and established practice who are exposed to such exceptional cases
-
- Includes an internationally recognized group of experts who discuss the technical approaches, success rates, complication rates, and special precautions needed to achieve optimal outcomes
-
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Catheter Ablation of Cardiac Arrhythmias in Children and Patients with Congenital Heart Disease by Edward P. Walsh, George F. Van Hare, Paul Khairy, Mohammad Shenasa in PDF and/or ePUB format, as well as other popular books in Medicine & Cardiology. We have over one million books available in our catalogue for you to explore.
Information
Part IIntroductory Concepts
Chapter 1DEVELOPMENT AND MORPHOLOGIC ASPECTS OF THE CARDIAC CONDUCTION SYSTEM IN NORMAL HEARTS AND CONGENITAL HEART DEFECTS
DOI: 10.1201/9781003082101-2
Introduction
There is an inherent beauty to the cardiac conduction system. Its journey to creation is a remarkably complex process beginning in the second week of embryological development. By birth, there is a mature network of conduction tissue responsible for initiating and maintaining the cardiac heartbeat. Abnormalities in cardiogenesis, which result in congenital heart defects, produce various conduction system anomalies. Cardiologists, electrophysiologists, and surgeons must have an in-depth knowledge of the cardiac conduction system in normal and congenital hearts. In the following chapter, we provide insight into this complexity as we discuss the embryological origins and morphological aspects of the conduction system in both normal hearts and those with congenital heart defects.
Embryological Development of the Cardiac Conduction System
Cardiac development is initiated at the end of the second week of embryological development, and by approximately 3 weeks, fusion of two endocardial tubes occurs to create the primary heart tube. This primary tube is a single endocardial tube with two caudal inlets (often referred to as venous poles) and one cranial outlet. The primary heart tube is composed of five key components; the sinus venosus, primitive atrium, primitive ventricle, bulbus cordis (conus), and truncus arteriosus (Figure 1.1). The sinus venosus receives inflow from both sinus horns (left and right), with the right horn incorporated into the right atrium and the left horn developing into the coronary sinus. The primitive atrium develops into the definitive left and right atria, with the left ventricle formed from the primitive ventricle. The right ventricle is formed from the bulbus cordis, which also contributes to the aortic outflow tract. The truncus arteriosus creates the ascending aorta and pulmonary trunk. As this primary tube develops, it elongates by adding cells to the heart tube from the secondary heart field. The secondary heart field is critical for establishing the cardiac outflow tract and right ventricle. It also contributes to the formation of the proepicardium. Later in development, the cardiac neural crest cells travel from the neural tube through the aortic arches and terminally arrive at the outflow tract. These cells are critical in ensuring the septation of the ventricles and outflow tract. Further, they serve a critical role in forming the anterior parasympathetic plexus. This plexus contributes to cardiac innervation and, subsequently, the regulation of heart rate. Although previously an area of debate, it is essential to recognize that the cardiac conduction system arises from the myocardium and does not show any lineage to cardiac neural crest cells.
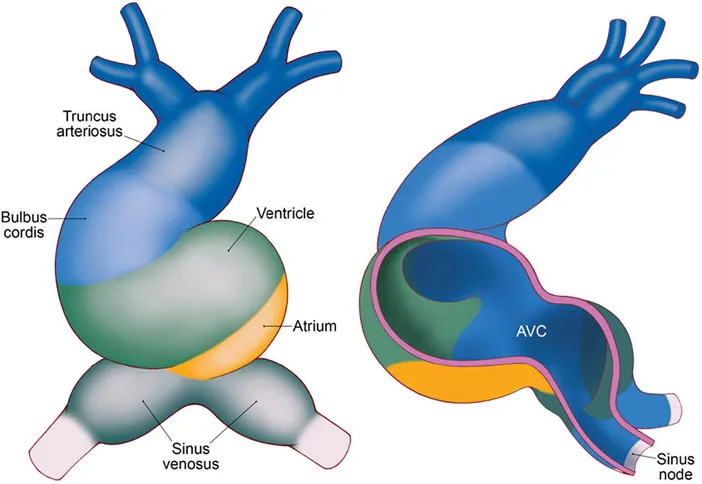
At 3 weeks, the heart tube is rather primitive in its electrical conduction. Critical components of mature conduction in the normally developed heart are not present, and consequently, pacemaker activity is slow and unidirectional, and results in a peristaltic-like contraction, traveling craniallyfrom the venous pole of the cardiac tube. Recording of electrical activity at this stage reveals a sinusoidal ECG. However, as the myocardium begins to evolve and develop, conduction velocity increases differentially, and conduction in the cardiac tube is no longer uniform. Areas of slow, primitive conduction velocity remain (sinus venous, the atrioventricular canal, and the outflow tract), enabling sphincter-like antegrade blood flow without the need for valves, which develop later from the endocardial cushions.
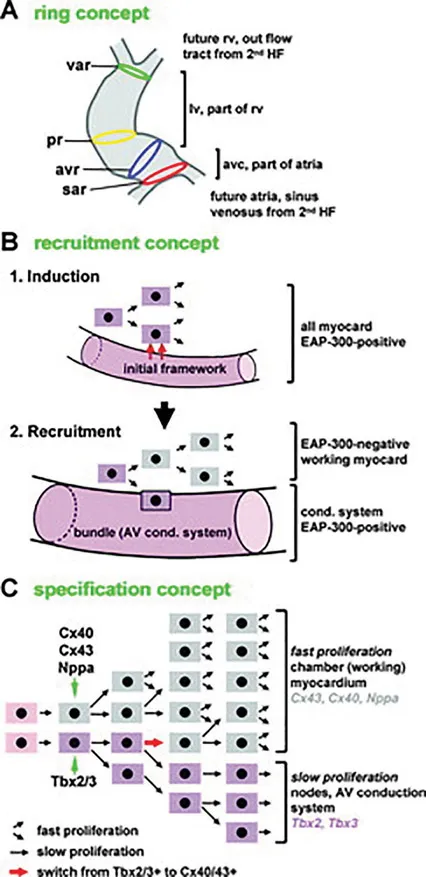
Three proposed models have described the contribution of cardiac myocytes to the cardiac conduction system (Figure 1.2). The first was the ring theory based upon the early observation (Reprinted with permission from Christoffels VM, Moo rman AF. Development of the cardiac conduction system: why are some regions of the heart more arrhythmogenic than others? Circulation: Arrhythmia and Electrophysiology. 2009 1;2: 195–207.) that the conduction system tended to form within the four constriction points in the normal looped heart (D-loop). This model postulated that these four rings existed in the heart before looping and subsequently developed into the sinus node, atrioventricular node, His bundle, and bundle branches.1,2 However, this model has since been discredited. Two other similar models are termed the “recruitment model”3 and the “early specification model.”4 In the recruitment model, cardiac progenitor cells create a conduction or myocyte cell. As embryological growth continues, these cells hold bipotential traits and can continue to create conduction cells, resulting in ongoing recruitment. Conversely, in the early specification model, a cardiac progenitor cell can separate into a conduction or myocyte cell, but once a cell becomes dedicated to one of these outcomes, they remain divergent from each other. The early specification model is favored presently.
The genetic transcription of the cardiac conduction system is complex and well beyond the scope of this book chapter. However, the following sections will provide a brief review with further detailed information found in well-written reviews.5–7
Sinus Node
The dominant pacemaker originates from the venous pole, and this continues until the formation of the sinus node (SN), which is readily identified by the eighth week of fetal life.8 Specifically, the sinus node arises from the sinus venous right horn. The genetic transcription that leads to SN development is a complex pathway of signaling and regulation. It is the most extensively studied process compared to the other conduction system elements. This SN arises from specialized Tbx18+/Nkx2.5- progenitors within the venous pole. These progenitor cells express Isl1, Shox2, and Tbx3, which play a critical role in regulating and expressing other transcription factors. In particular, they ensure pacemaker activity is confined to the venous pole (particularly restricting to the right side) and suppress atrialization of tissue, particularly preventing the differentiation towards a working myocardium phenotype.
Atrioventricular Node
Unlike the sinus node, the mechanistic development and transcription pathways for the atrioventricular node (AVN) are poorly delineated, largely because it is a heterogeneous structure composed of transitional cells, atrial inputs, and compact nodal cells. Lineage tracing studies of the AVN show that it is created from the embryonic atrioventricular canal (AVC). Primitive AVN cells appear as early as embryonic day 9.5, shortly after chamber differentiation. The AVC retains its slow conducting phenotype throughout development. Tbx2, Tbx3, and Bmp2 are among the most important regulators of the AVC and play a critical role in developing the AVC and AVN. Like the SN, these factors regulate and express other factors that ultimately prevent myocardial gene programming, ensuring the AVC retains its primitive, slowly conducting phenotype. It is the formation of the annulus fibrosis (approximately embryonic day 12) that insulates the atrial and ventricular myocardium.
Proximal Ventricular Conduction System (His and Left and Right Bundles)
The peripheral conduction system is derived from ventricular myocardial precursors and specifically arises from the ventricular septum crest. In contrast to the AVN, proximal conduction tissue is not created from the Tbx+ cells of the AVC. The His bundle originates from the Tbx3+ primary interventricular ring, specifically the ventricular septal part with the bundle branches created from the Tbx3+ subendocardial trabeculae. Tbx5 and Nkx2–5 are critical regulators in the proximal conduction system and bundle branch formation. These factors are critical in creating fast conduction properties of the conduction tissue and ensuring there is inhibition of myocardial gene programming.
Purkinje Fibers (Peripheral Conduction System)
Beginning with early studies in avian embryos, there has been considerable debate about whether the Purkinje network originates from specialized precursor tissue within the ventricles or by differentiation from a common ventricular myocardium. While evidence has been presented for both, the majority of evidence suggests that the Purkinje fibers (PF) share a common progenitor with contractile cardiomyocytes, and they are derived from the trabecular myocardium. It was demonstrated in avian models that PF share a common cellular progenitor with the working myocardium and that the recruitment ...
Table of contents
- Cover Page
- Half Title Page
- Title Page
- Copyright Page
- Dedication Page
- Contents Page
- Foreword Page
- Preface Page
- Editors Page
- Contributors Page
- Part I Introductory Concepts
- Part II Catheter Ablation of Supraventricular Arrhythmias in Young Patients with Structurally Normal Hearts
- Part III Ablation of Supraventricular Tachycardia in Congenital Heart Disease
- Part IV Catheter Ablation of Ventricular Arrhythmias in Pediatrics and Congenital Heart Disease
- Part V Miscellaneous Topics
- Index