
eBook - ePub
Neuroimaging and Psychosocial Addiction Treatment
An Integrative Guide for Researchers and Clinicians
This is a test
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Neuroimaging and Psychosocial Addiction Treatment
An Integrative Guide for Researchers and Clinicians
Book details
Book preview
Table of contents
Citations
About This Book
Using an innovative translational approach between the work of experimental scientists and clinical practitioners this book addresses the current, modest, understanding of how and why addiction treatment works. Through bridging this gap it provides a critical insight into why people react as they do in the context of addiction treatment.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Neuroimaging and Psychosocial Addiction Treatment by Sarah W. Feldstein Ewing, Katie Witkiewitz, Francesca M. Filbey, Sarah W. Feldstein Ewing,Katie Witkiewitz,Francesca M. Filbey in PDF and/or ePUB format, as well as other popular books in Psicologia & Psicologia clinica. We have over one million books available in our catalogue for you to explore.
Information
Topic
PsicologiaSubtopic
Psicologia clinicaSection II
Translational Approaches with Adults
3
Using Clinical Neuroscience to Understand Addiction Treatment
Joseph P. Schacht and Kent E. Hutchison
I. Background
In the past several decades, the advent of clinical neuroscience has fundamentally changed the course of research into treatments for adult substance use disorders (SUDs) (Heilig et al., 2010; Karoly et al., 2013). Clinical neuroscience methods have leveraged knowledge from animal models of addiction, which suggest that SUDs can be understood as dysfunction in two fundamental brain systems that represent motivated behavior/reward and inhibitory control (Kalivas & Volkow, 2005). These models suggest that motivated, goal-directed behavior is represented in the brain by an interconnected network of brain areas that rely primarily on dopamine and glutamate signaling (see Chapter 1). Dopamine transmission along this pathway underlies the acutely rewarding effects of all substances of abuse and the attribution of incentive salience to stimuli associated with these substances; after the onset of SUDs, excessive dopamine signaling is believed to underlie craving for substances (Berridge & Robinson, 1998; Wise, 1988). However, SUDs are also characterized by dysfunction of a neural network that underlies control over compulsive actions such as substance use (Bechara, 2005). Animal models of SUDs suggest that the transition from initial substance use, to withdrawal and negative affect, and ultimately to compulsive, uncontrolled substance use is characterized by hyperactivation of the reward network and hypoactivation of the inhibitory control network (Koob & Volkow, 2010).
This chapter focuses on research that has used fMRI to study adult SUD treatment outcomes in the context of the reward and inhibitory control networks outlined in Figure 3.1. Based on the conceptual framework that has evolved over the last two decades, treatments may influence brain activation in areas associated with either or both of these networks, which, in turn, are likely to predict treatment success and relapse propensity.
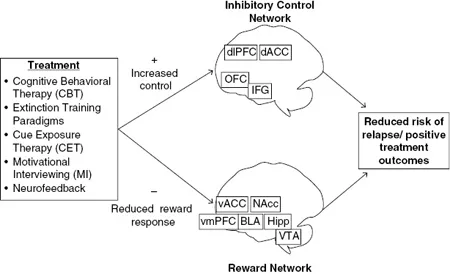
Figure 3.1 Reward and inhibitory control networks believed to underlie positive treatment outcomes. See text for brain area abbreviations.
II. Psychosocial Treatment Effects on Neuroimaging Paradigms
A. Overview of extant studies
Significant attention has been devoted to evaluating whether treatments for adult SUDs affect brain activation elicited by neuroimaging paradigms. The majority of studies have employed pharmacological interventions to test this hypothesis. However, at least seven studies have examined the effects of psychosocial treatment, either alone or in combination with pharmacological intervention (see Table 3.1). To date, these studies have focused exclusively on cue reactivity paradigms. Cue reactivity is one of the longest-studied phenomena in substance use research, and several recent meta-analyses (Chase et al., 2011; Engelmann et al., 2012; Schacht et al., 2013a) and reviews (Jasinska et al., 2014; Yalachkov et al., 2012) summarize the neuroimaging literature on this, including a variety of individual difference variables that affect it. Most studies of psychosocial treatments have focused on small samples of alcohol- and nicotine-dependent individuals and have evaluated the effects of relatively brief treatments. Despite the increased statistical power they offer, pre-/post-treatment designs have not been widely used, nor have placebo treatments (e.g., waitlist controls or supportive psychotherapy) been employed as a statistical control. Nonetheless, across studies, the most consistent effect reported has been treatment-induced reduction of cue-elicited activation of the ventral ACC, a core component of the reward network. Interestingly, this effect has been reported for alcohol-, nicotine-, and cocaine-dependent subjects. At least two have found that psychosocial treatments reduced cue-elicited activation of other reward network areas, including the ventral striatum and insula. The ventral striatum finding is concordant with pharmacological treatment outcomes for alcohol cue reactivity, in which treatment-induced reduction of cue-elicited ventral striatal activation has been the most consistently reported finding (Schacht et al., 2013a).
Table 3.1 Psychosocial treatment effects on cue-elicited brain activation (as organized by substance)
| First author, year | Substance | Type and length of treatment | Active N | Control N | Scans | Brain areas in which treatment reduced activation | |||||||
| VS | DS | BLA | Ins | ACC | IFG | dPFC | IPL | ||||||
| Schneider, 2001 | Alcohol | CBT (15 sessions) + doxepin × 21 days | 10 | n/a | Pre/post | ||||||||
| McClernon, 2007 | Nicotine | Extinction-based smoking cessation × 14–28 days | 16 | n/a | Pre/post | L, R | |||||||
| Feldstein Ewing, 2011 | Alcohol | MI (1 session; change vs. counterchange talk) | 13 | n/a | Post | L | L | ||||||
| Vollstädt-Klein, 2011 | Alcohol | CET (9 sessions) × 21 days | 15 | 15 | Pre/post | L | L | L | L, R | L, R | L, R | ||
| Janse Van Rensburg, 2012 | Nicotine | Cardiovascular exercise (1 10-m session) | 20 | 20 | Post (crossover) | ||||||||
| Li, 2013 | Nicotine | Real-time neurofeedback (1 session) | 10 | n/a | Pre/post | M | |||||||
| Prisciandaro, 2013 | Cocaine | CET (2 sessions) + DCS × 7 days | 10 | 15 | Pre/post | *L, R | *R | *L | *R | ||||
| *Indicates areas in which cue-exposure therapy reduced cue-elicited activation relative to the pre-treatment scan. DCS did not reduce activation in any area and potentiated it in the occipital cortex. Abbreviations: CBT = Cognitive Behavioral Therapy; MI = Motivational Interviewing; CET = Cue-Exposure Therapy; DCS = D-cycloserine; VS = ventral striatum; DS = dorsal striatum; BLA = basolateral amygdala; Ins = insula; ACC = anterior cingulate cortex; IFG = inferior frontal gyrus; dPFC = dorsal prefrontal cortex; IPL = inferior parietal lobule; L = left; R = right; M = medial. | |||||||||||||
B. Treatment effects on the neural substrates of cue reactivity
The first published study of treatment effects on alcohol cue-elicited activation demonstrated some of the methodological issues inherent to this line of research. Among ten treatment-seeking individuals with alcohol dependence, Schneider and colleagues (Schneider et al., 2001) tested the effects of three weeks of Cognitive Behavioral Therapy (CBT; five hours per week of relapse prevention, stimulus control, and psycho-education) combined with the tricyclic antidepressant doxepin, a serotonin and norepinephrine reuptake inhibitor, on activation elicited by olfactory alcohol cues. Before treatment was initiated, patients demonstrated cue-elicited activation of the right amygdala and left cerebellum that was not present in a group of matched controls. After treatment, activation of these regions was not present in either group. However, the difference between time points was not statistically tested; further, it was not possible to disentangle the effects of doxepin and CBT, nor those of time, as no placebo was used to control either the pharmacological or the psychosocial intervention.
The Schneider study essentially tested the effects of treatment as usual in the German alcohol treatment system on cue-elicited activation, but three more recent studies have made more theoretically driven attempts to modulate this phenomenon through different extinction training paradigms. McClernon and colleagues (McClernon et al., 2007) explored the effects of an extinction-based smoking cessation program in which 16 treatment-seeking, nicotine-dependent cigarette smokers switched to reduced nicotine cigarettes for two to four weeks while wearing a transdermal nicotine patch before ultimately attempting to quit smoking. Because the nicotine patch maintained a steady blood level of nicotine, subjects did not experience nicotine withdrawal when they switched to the reduced nicotine cigarettes, but their nicotine intake was no longer contingent on smoking behavior or cues. Region-of-interest (ROI) analyses focused on reward-related areas (ACC, PFC, amygdala, hippocampus, striatum, thalamus, and insula). Relative to a pre-treatment scan, activation elicited by visual nicotine cues was reduced bilaterally in the amygdala after treatment, although this activation rebounded somewhat after the quit attempt was made; other ROIs did not display treatment-related reductions in activation. Thalamic cue-elicited activation was also reduced before the quit attempt but only for subjects who maintained one month of abstinence after the quit attempt.
Vollstädt-Klein and colleagues examined the effects of a different method for ablating the contingency between cues and the acute effects of a substance (Vollstadt-Klein et al., 2011). Thirty abstinent, alcohol-dependent patients who were engaged in an intensive outpatient treatment program (IOP) in Germany were randomly assigned to either three weeks (nine sessions) of Cue-Exposure Therapy (CET), consisting of both real exposure to alcoholic beverages and imaginal exposure to situations involving cues that were judged likely to precipitate relapse, or treatment as usual in the IOP....
Table of contents
- Cover
- Title
- Copyright
- Contents
- List of Tables
- List of Figures
- Acknowledgments
- Preface
- About the Editors
- List of Contributors
- Section I: Introduction
- Section II: Translational Approaches with Adults
- Section III: Translational Approaches with Adolescents
- Section IV: Epilogue
- Index