![]()
1
Hyperosmolar Therapy in Traumatic Brain Injury
Haley Goodwin Gibbs, Jose Guzman Garcia, and Ron Neyens
INTRODUCTION
Severe brain injury results in a complex secondary injury cascade, with ensuing cerebral edema and elevated intracranial pressure (ICP). This process is a common contributor to permanent neurological sequelae and death. Cerebral edema is primarily manifested as 2 distinct physiological processes: vasogenic (eg, capillary permeability) and/or cytotoxic (eg, cellular apoptosis).1 Osmotherapy is designed to create an osmotic gradient across the blood-brain barrier (BBB), resulting in an intended reduction of brain water content.
Osmotherapy has a long-standing history, with concentrated electrolyte solutions shown to decrease ICP and brain bulk in the early 1900s. It was not until the 1950s before osmotic formulations were routinely used clinically as a therapeutic tool in the management of intracranial hypertension. Initially, formulations of urea were standard therapy, but adverse effects and observations of intracranial pressure rebound limited its use, driving a transition to glycerol and sorbitol in the 1960s, followed by mannitol in the late 1960s and hypertonic saline in the 1990s.2 The latter 2 osmotic agents now stand as guideline-based recommendations and a major component of neurocritical care treatment strategies for cerebral edema and intracranial hypertension.
MECHANISM
The exact mechanism of ICP reduction with osmotherapy remains ill-defined yet potentially involves several characteristics. The major therapeutic impact is felt to be a complex and dynamic result of osmotic dehydration and rheologically induced cerebrovascular vasoconstriction with reduced cerebral blood volume (CBV).
Osmotic Dehydration
The BBB is a highly selective permeability barrier separating the brain extracellular fluid from the circulating blood. It is composed of endothelial cells, connected by high-density cellular tight junctions. It serves as a self-protective mechanism allowing passive transport of water and small lipid-soluble molecules, as well as active transport of molecules necessary for optimal neuronal function.3 It relies on the osmotic principle that the BBB allows transport of water from compartments with low osmolality to those with higher osmolality. An induced high-osmole concentration gradient mobilizes water from the interstitial and intracellular compartments of the brain into the intravascular compartment, therefore reducing brain water content, mass effect, and ICP. Clinical practice has evolved to using mannitol and hypertonic sodium solutions as they are more effective osmoles than urea, glycerol, and sorbitol. Therefore, they display low permeability across the BBB, referred to as its reflection coefficient (σ), which is an index of the effectiveness of a solute in generating an osmotic driving force (σ = 1 for hypertonic sodium solutions and σ = 0.9 for mannitol on a scale of 0–1, with the most effective being 1). The osmotic model assumes an intact BBB, expressing low hydraulic conductivity.4 The degree of BBB disruption is not readily determined clinically in the neurologically injured patient. Therefore, the osmotic dehydration effect may occur more effectively in normal rather than injured or impaired brain tissue; however, the clinical relevance remains debatable and ill-defined.5
Rheology and Vascular Reactivity
The brain has little capacity to store oxygen, with its survivability dependent on an adequate cerebral blood flow (CBF) and cerebral oxygen delivery (CDO2). In normal physiological conditions, homeostatic cerebral autoregulation is in place to ensure that supply (ie, CBF and CDO2) is coupled to meet demand (ie, cerebral metabolic rate for oxygen consumption), despite variations in cerebral perfusion pressure (CPP). This occurs via a tight regulation of cerebral vascular resistance, stimulated by various interrelated processes involving pressure, chemical, and electrical gradients.6 The rheological principle of osmotherapy relies on its ability to increase red blood cell deformity resulting in reduced blood viscosity, independent of changes in hematocrit.2 It is proposed that this promotes compensatory vasoconstriction of both arterioles and venules on the surface of the brain to maintain homeostatic CBF and thus reduces CBV and resultant ICP.7 This model assumes intact vascular reactivity and has been challenged in the pathology of acute brain injury.8
Pleotropic
Several other proposed mechanisms exist, including immunomodulatory, free radical scavenging, and neuroendocrine effects.9,10,11 With unclear clinical relevance, hypertonic sodium solutions may assist in preserving the BBB by restoring normal resting membrane potential, which is not observed with mannitol.12
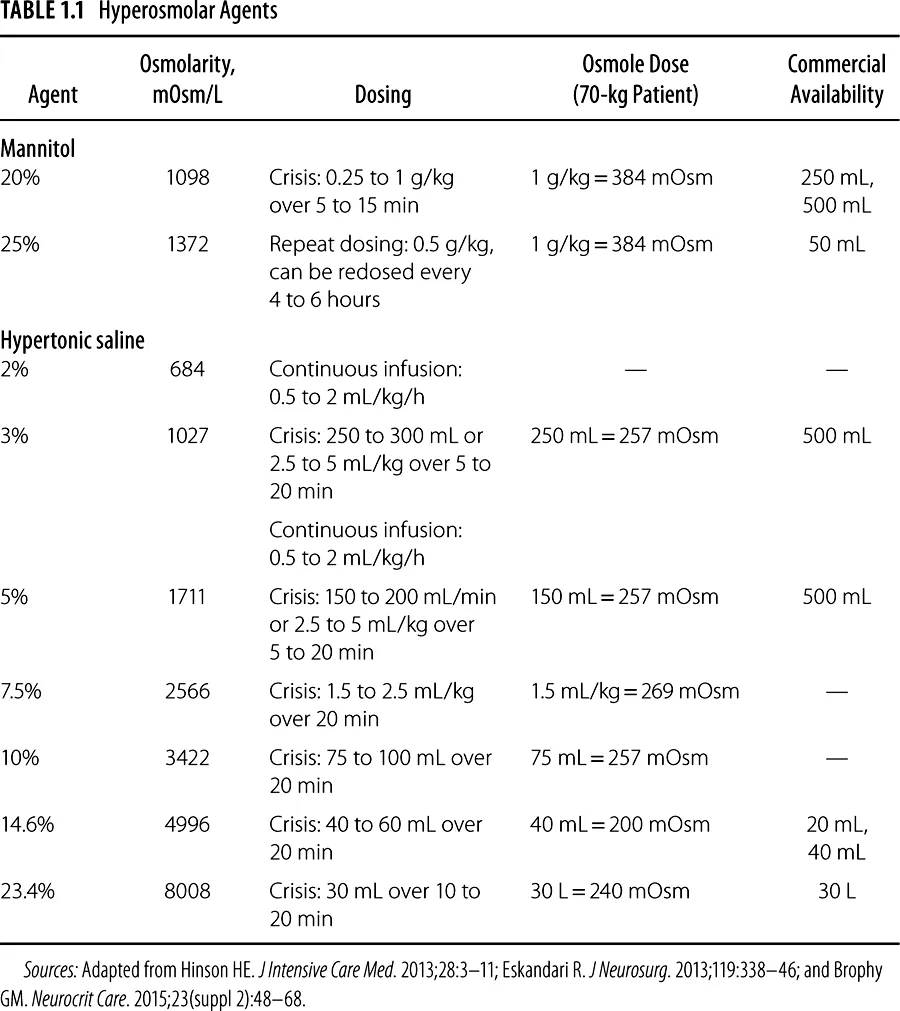
HYPEROSMOLAR AGENTS
There are several different concentrations of mannitol and hypertonic sodium solutions used in clinical practice. The agents and doses used display variable osmolarities but are believed to yield a therapeutic response via similar principles. For osmotic agents routinely used in clinical practice, see Table 1.1.
Pharmacokinetics and Pharmacodynamics
An optimal dose-response relationship has not been clearly defined, and many of the relevant comparative studies have not used equiosmolar dosing strategies. Based on initial clinical observations, an osmotic gradient of 5 to 10 mOsmol/kg was considered necessary for therapeutic efficacy.13,14 However, it remains incompletely understood as it is clear that hypertonic sodium solutions effectively reduce ICP, with relatively small total osmole doses (eg, 23.4% 30 mL = 240 mOsm), even in the face of high serum osmolarity (eg, >320 mOsm/L).15 In addition, it is a common clinical practice that doses of mannitol 20% and hypertonic sodium solutions are often not equiosmolar, yet both are effective at reducing elevated ICPs. An equiosmolar dose of 1 g/kg 20% mannitol is approximately 5.3 mL/kg 3% saline, 3.2 mL/kg 5% saline, 2.1 mL/kg 7.5% saline, 1.6 mL/kg 10% saline, 1.1 mL/kg 14.6%, and 0.69 mL/kg 23.4% saline.
For both mannitol and hypertonic sodium solutions, the effect on ICP tends to be biphasic. The initial rapid ICP reduction, occurring within minutes, is possibly related to the previously mentioned rheological and cerebral vascular reactivity principles. However, the intensity of effect may be dependent on the mean arterial blood pressure (MAP). It has been shown that in the face of elevated blood pressure, an impressive rise in CBF occurred, particularly in the noninjured regions of the brain. However, the CBV was not significantly reduced. Therefore, it has been proposed that osmotherapy-reduced viscosity and reflexive vasoconstriction may be lost at high perfusion pressures (eg, MAPs ≥ 120–130 mm Hg), exceeding the upper limits of autoregulation and reactivity of cerebral resistance arterioles. In effect, they may be maximally vasoconstricted and unable to further respond to reduced viscosity.8 Although unproven, compensatory vasoconstriction may remain intact at moderate perfusion pressures, assuming autoregulation remains intact.
The later phase, which is delayed 15 to 30 minutes after drug administration, is related to osmotic principles and cellular dehydration. Pharmacokinetic data specifically with hypertonic saline are lacking, but it is observed to be similar to mannitol, with initial effects within minutes, a peak between 15 and 120 minutes, and a duration of response up to 4 to 6 hours.16 The hydration of brain tissue with osmotherapy persists longer than one would expect given the relatively short serum half-life of the osmotically active agents.
ADMINISTRATION AND STORAGE
Access and Infusion Considerations
Mannitol doses for the treatment of elevated ICP can be infused over 5 to 30 minutes through a peripheral or central line.17,18 The ability to infuse peripherally makes mannitol an ideal agent in the emergency department or in patients without central access. Rapid infusion over a few minutes produces maximal effects to decrease ICP. Mannitol solutions with concentrations greater than 15% have a tendency to crystallize and therefore require administration through an in-line filter.17,18
Hypertonic saline solutions with concentrations greater than 3% are to be administered via a central venous catheter, although the infusion of 3% saline solutions through peripheral venous access at infusion rates of up to 50 mL/h has been associated with a low risk of infusion-related complications.19 Because rapid infusions of 23.4% can cause hypotension, 30- to 60-mL doses can be administered over 20 minutes or via slow intravenous push as tolerated by the patient. Doses and infusion rates for boluses of lesser concentrated hypertonic saline solutions are patient specific but are generally between 250 and 500 mL and can be infused rapidly via an infusion pump.
As the intraosseous route of administration has been promoted for emergency settings, such as in advanced cardiac life support, it begs the question of whether there is a role to play in neurological emergencies, especially when laying the patient flat for line placement is not possible due to the patient’s clinical condition. The effective administration of 7.5% sodium chloride/6% dextran 70 via the intraosseous route has been established in military medicine studies. In addition, Chavez-Negrete et al20 showed that intraosseous administration of 250 mL of 7.5% sodium chloride/6% dextran 60 was an effective initial treatment for hemorrhagic shoc...