
- 235 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
An Introduction to Surfactants
About this book
Surfactants are surface active agents, molecules that have a significant role in emulsions, suspensions, and foams. They find widespread application in personal care, cosmetics, pharmaceuticals, agrochemicals and the food industry. The main objective of this graduate level textbook is to present an overview of the classification, physical properties, phase behavior, their effects and applications of surfactants, e.g. as emulsifiers, foam stabilizer, in nano- and microemulsions and as wetting agents.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1 General introduction
Surface active agents (usually referred to as surfactants) are amphiphilic or amphipathic molecules consisting of a non-polar hydrophobic portion, usually a straight or branched hydrocarbon or fluorocarbon chain containing 8–18 carbon atoms, which is attached to a polar or ionic portion (hydrophilic). The term amphiphilic originates from the Greek word “amphi”, meaning “both” and the term relates to the fact that all surfactant molecules consist of at least two parts, one which is soluble in a specific fluid, e.g. water (the hydrophilic part) and one which is insoluble in water (the hydrophobic part). The hydrophilic portion can be nonionic, ionic or zwitterionic, accompanied by counter ions in the last two cases. The hydrocarbon chain interacts weakly with the water molecules in an aqueous environment, whereas the polar or ionic head group interacts strongly with water molecules via dipole or ion-dipole interactions. It is this strong interaction with the water molecules which renders the surfactant soluble in water. However, the water molecules avoid contact with the hydrophobic chain and their cooperative action of dispersion and hydrogen bonding tends to squeeze the hydrocarbon chain out of the water by accumulation at interfaces and association in solution to form aggregate units referred to as micelles. In the latter case, the surfactant hydrophobic groups are directed towards the interior of the aggregate and the polar head groups are directed towards the solvent. These micelles are in dynamic equilibrium and the rate of exchange between a surfactant molecule and the micelle may vary by orders of magnitude, depending on the structure of the surfactant molecule. The balance between hydrophilic and hydrophobic parts of the molecule (referred to as the hydrophilic-lipophilic balance, HLB) gives these systems their special properties such as adsorption at interfaces and formation of self-assembly structures.
Surfactants have the property of adsorbing onto the surfaces or interfaces of the system and of altering the surface or interfacial free energy of those surfaces or interfaces. The driving force for surfactant adsorption is the lowering of the free energy of the phase boundary. The interfacial free energy per unit area is the amount of work required to expand the interface. This interfacial free energy, referred to as surface or interfacial tension, γ, is given in mJm–2 or mNm–1. Adsorption of surfactant molecules at the interface lowers the surface tension γAW (at the air/liquid interface) or interfacial tension γOW (at the oil/water interface) and the higher the surfactant adsorption (i.e. the more dense the layer is) the larger the reduction in γ. Surfactants also adsorb at the solid/liquid interface and this causes a reduction in the solid/liquid interfacial tension, γSL. The degree of surfactant adsorption at the interface depends on surfactant structure and the nature of the two phases that meet the interface [1], [2], [3], [4],[5],[6].
dp n="14" folio="2" ?
When studying surfactants one should consider two main phenomena:
- Interfacial effects that relates to the adsorption and orientation of the molecules at various interfaces. This requires accurate measurements of the adsorption and orientation of the surfactant ions or molecules.
- Colloid stability that relates to the effect of surfactants on stabilization of various disperse systems, e.g. emulsions, suspensions, foams, nanoemulsions and microemulsions. It should be mentioned that this subdivision is only for convenience since colloid and interface science are one and the same subject of study. All colloid stability phenomena are related to the interfacial phenomena.
Surfactants find application in almost every chemical industry of which the following may be worth mentioning: detergents, paints, dyestuffs, cosmetics, pharmaceuticals, agrochemicals, fibers, plastics, etc. Moreover, surfactants play a major role in the oil industry, for example in enhanced and tertiary oil recovery. In the latter case surfactant micellar systems and microemulsions are used to recover oil from micro-pores that has been entrapped as a result of capillary forces. They are also occasionally used for environmental protection, e.g. in oil slick dispersants. The spilled oil from tankers and oil wells is emulsified using surfactants and the resulting emulsion is separated and then the system is demulsified to recover the oil. Therefore, a fundamental understanding of the physical chemistry of surface active agents, their unusual properties and their phase behavior is essential for most industrial chemists. In addition, an understanding of the basic phenomena involved in the application of surfactants, such as in the preparation of emulsions and suspensions and their subsequent stabilization, in nanoemulsions, in microemulsions, in wetting spreading and adhesion, etc., is of vital importance in arriving at the right composition and control of the system involved [1, 2]. This is particularly the case with many formulations in the chemical industry mentioned above.
It should be stated that commercially produced surfactants are not pure chemicals, and within each chemical type there can be tremendous variation. This can be understood, since surfactants are prepared from various feed stocks, namely petrochemicals, natural vegetable oils and natural animal fats. It is important to realize that in every case the hydrophobic group exists as a mixture of chains of different lengths. The same applies to the polar head group, for example in the case of polyethylene oxide (the major component of nonionic surfactants) which consists of a distribution of ethylene oxide units. Hence, products that may be given the same generic name could vary a great deal in their properties and the formulation chemist should bear this in mind when choosing a surfactant from a particular manufacturer. It is advisable to obtain as much information as possible from the manufacturer, such as the distribution of alkyl chain length, distribution of the polyethylene oxide chain and also the properties of the surfactant chosen such as its suitability for the job, its batch to batch variation, its toxicity, etc. The manufacturer usually has more information on the surfactant
than that printed in the data sheet, and in most cases such information is given on request.
This book gives an introduction to surfactants, their solution properties, adsorption at various interfaces and their applications in various disperse systems. Chapter 2 gives a general classification of surfactants based on the nature of the head group (anionic, cationic, zwitterionic and nonionic). Description of some specialized molecules, such as fluorocarbon and silicone surfactants (referred to as superwetters), and sugar-based surfactants is also given. Naturally occurring surfactants that are used in the food industry and pharmaceuticals are also described. A section will be devoted to polymeric surfactants. The latter are particularly important for stabilization of disperse systems. Chapter 3 deals with the unusual properties of surfactant solutions that show abrupt changes at a particular concentration that is related to the formation of aggregate units referred to as micelles. This concentration that is referred to as the critical micelle concentration (CMC) depends on the structure and nature of the surfactant molecule. The different self-assembly structures that are produced in surfactant solutions are described in terms of their structures and phase behavior. Chapter 4 describes the process of surfactant adsorption at the air/liquid (A/L), liquid/liquid (L/L) and solid/liquid (S/L) interfaces. A thermodynamic treatment of the process of surfactant adsorption is given. This treatment can be applied for the reversible adsorption of the surfactant molecules whereby an equilibrium is established when the rate of adsorption becomes equal to the rate of desorption. Such thermodynamic treatment cannot be applied for the adsorption of polymeric surfactants since in this case the adsorption process is not reversible. For that case statistical thermodynamic treatment of the process of adsorption can be applied. The experimental techniques that can be applied for measuring surfactant adsorption at various interfaces are briefly described. Understanding the process of surfactant adsorption at various interfaces is very important in their application. For example, the process of wetting and spreading on various interfaces is determined by the adsorption of surfactant molecules at the A/L and S/L interfaces. Adsorption at the L/L interfaces determines the process of emulsification and emulsion stability. The same applies for nanoemulsions and microemulsions. Chapter 5 describes the use of surfactants as emulsifiers; particular attention is given to the methods that can be applied for selection of emulsifiers for a given oil used in the emulsion. The role of surfactants in stabilizing the emulsion against flocculation, Ostwald ripening and coalescence is also described at a fundamental level. Chapter 6 describes the use of surfactants as dispersants for suspensions. The process of dispersion of powders in liquids is described in terms of wetting, dispersion and stabilization of the resulting suspension against flocculation. The process of Ostwald ripening (crystal growth) and the role of surfactants is described at a fundamental level. Chapter 7 deals with surfactants in foam formation and stabilization. The theories of foam stabilization and the role of surfactants are described. Application of surfactants in formulation of nanoemulsions is described in Chapter 8. Nanoemulsions are a special class of emulsions with droplet size in the range 20–200 nm. Their main
advantages in formulation are described. The various methods that can be applied for preparation of nanoemulsions are described. Chapter 9 describes microemulsions and the origin of their thermodynamic stability. The various methods that can be applied for formulation of microemulsions are described. The use of surfactants as wetting agents will be described in Chapter 10. Particular attention is given to the fundamentals of wetting and spreading with particular reference to surfactants that can be used as wetting agents. The final Chapter 11 will deal with application of surfactants in various industries: Cosmetics - Pharmaceuticals - Agrochemicals - Paints and Coatings - Detergency - Oil Recovery.
It should be mentioned that this book is written for graduate students and scientists who are beginners in the subject. As far as possible, the subject is dealt with at a fundamental level without too much detail. For more comprehensive understanding of the subject of surfactants, the reader can refer to other texts that are given in the reference list.
References
[1]
Th. F. Tadros (ed.), Surfactants, Academic Press, London,1984.
[2]
M. R. Porter, Handbook of Surfactants, Chapman and Hall, Blackie, USA, 1994.
[3]
K. Holmberg, B. Jonsson, B. Kronberg and B. Lindman, Surfactants and Polymers in Solution, second edition, John Wiley and Sons, Ltd., Chichester, UK, 2003.
[4]
Th. Tadros, Applied Surfactants, Wiley-VCH, Germany, 2005.
[5]
M. J. Rosen and J.T. Kunjappu, Surfactants and Interfacial Phenomena, John Wiley and Sons, USA, 2012.
[6]
Th. Tadros (ed.), Encyclopedia of Colloid and Interface Science, Springer, Germany, 2013.
2 General classification of surfactants
A simple classification of surfactants based on the nature of the hydrophilic group is commonly used. Four main classes may be distinguished, namely anionic, cationic, amphoteric. and nonionic [1], [2]. A useful technical reference is McCutcheon [3], which is produced annually to update the list of available surfactants. A recent text by van Os et al. [4] listing the physicochemical properties of selected anionic, cationic and nonionic surfactants has been published by Elsevier. Another useful text is the Handbook of Surfactants by Porter [5]. It should also be mentioned that a fifth class of surfactants, usually referred to as polymeric surfactants, has been used for many years for preparation of emulsions and suspensions and their stabilization.
2.1 Anionic surfactants
These are the most widely used class of surfactants in industrial applications [5] [6] [7]. This is due to their relatively low cost of manufacture and they are practically used in every type of detergent. For optimum detergency the hydrophobic chain is a linear alkyl group with a chain length in the region of 12-16 C atoms and the polar head group should be at the end of the chain. Linear chains are preferred since they are more effective and more degradable than the branched chains. The most commonly used hydrophilic groups are carboxylates, sulfates, sulfonates and phosphates. A general formula may be ascribed to anionic surfactants as follows:
| Carboxylates: | CnH2n+1 COO– X+ |
| Sulfates: | 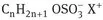 |
| Sulfonates: | 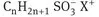 |
| Phosphates: | CnH2n+1 OPO(OH)O- X+ |
with n being the range 8-16 atoms and the counterion X+ is usually Na+.
Several other anionic surfactants are commercially available such as sulfosuccinates, isethionates (esters of isothionic acid with the general formula RCOOCH2–CH2–SO3Na) and taurates (derivatives of methyl taurine with the general formula RCON(R...
Table of contents
- Also of Interest
- Title Page
- Copyright Page
- Preface
- Table of Contents
- 1 General introduction
- 2 General classification of surfactants
- 3 Aggregation of surfactants, self-assembly structures, liquid crystalline phases
- 4 Surfactant adsorption at interfaces
- 5 Surfactants as emulsifiers
- 6 Surfactants as dispersants and stabilization of suspensions
- 7 Surfactants for foam stabilization
- 8 Surfactants in nanoemulsions
- 9 Surfactants in microemulsions
- 10 Surfactants as wetting agents
- 11 Industrial applications of surfactants
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access An Introduction to Surfactants by Tharwat F. Tadros in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Industrial & Technical Chemistry. We have over one million books available in our catalogue for you to explore.