
- 610 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
"This book contains overviews on technologically important classes of glasses, their treatment to achieve desired properties, theoretical approaches for the description of structure-property relationships, and new concepts in the theoretical treatment of crystallization in glass-forming systems. It contains overviews about the state of the art and about specific features for the analysis and application of important classes of glass-forming systems, and describes new developments in theoretical interpretation by well-known glass scientists. Thus, the book offers comprehensive and abundant information that is difficult to come by or has not yet been made public." Edgar Dutra Zanotto (Center for Research, Technology and Education in Vitreous Materials, Brazil)
Glass, written by a team of renowned researchers and experienced book authors in the field, presents general features of glasses and glass transitions. Different classes of glassforming systems, such as silicate glasses, metallic glasses, and polymers, are exemplified. In addition, the wide field of phase formation processes and their effect on glasses and their properties is studied both from a theoretical and experimental point of view.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1 Influence of Thermal Prehistory on Crystal Nucleation and Growth in Polymers
1.1 Introduction
1.2 State of the Art
1.2.1 Dependence of the Properties of Glass-forming Melts on Melt History
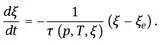
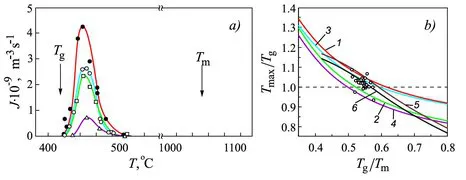
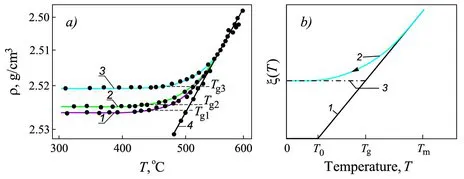
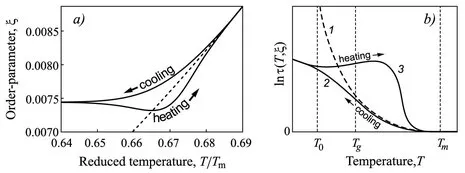
Table of contents
- Also of Interest
- Title Page
- Copyright Page
- Foreword
- Table of Contents
- Preface
- List of contributing authors
- 1 Influence of Thermal Prehistory on Crystal Nucleation and Growth in Polymers
- 2 Early Stages of Crystal Formation in Glass-forming Metallic Alloys
- 3 Crystalline and Amorphous Modifications of Silica: Structure, Thermodynamic Properties, Solubility, and Synthesis
- 4 The Main Silica Phases and Some of Their Properties
- 5 Chemical Structure of Oxide Glasses: A Concept for Establishing Structure–Property Relationships
- 6 Bubbles in Silica Melts: Formation, Evolution, and Methods of Removal
- 7 Regularities and Peculiarities in the Crystallization Kinetics of Silica Glass
- 8 Stress-induced Pore Formation and Phase Selection in a Crystallizing Stretched Glass
- 9 Crystallization of Undercooled Liquids: Results of Molecular Dynamics Simulations
- 10 Crystal Nucleation and Growth in Glass-forming Systems: Some New Results and Open Problems
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app