![]()
Part I: Introduction and outlook
dp n="20" folio="" ?
dp n="21" folio="" ?
![]()
Holger Loewe
1 Introduction and outlook
It has now been more than 15 years since the first International Conference on Microreaction Technology (IMRET) and microflow chemistry developed an established pathway for synthesizing high quality chemicals and products at an industrial scale.
Nevertheless, the so-called microreactor chemistry was not new at the time. It has been known since the late 1960s, but due to the lack of advanced fabrication technology to produce microreactors, the publications per year stayed very low until the early 1990s. Not only was the technology missing, but also an established journal on microchemistry, which hindered advancements in this field. Because of this, the first book was published in 2001 with the help of many enthusiastic researchers, who by then had been met with skepticism for this re-invented technology [1].
Today, there can be absolutely no doubt about the importance of microreaction technology. Several specialized journals have been established and a variety of books focusing on different challenges have already been written. Theories for computational microfluidics, as well as novel manufacturing techniques were developed, and new reaction pathways were published [2, 3]. These new pathways are summarized and introduced by Prof. Hessel under the topic of “Novel Process Windows”, leading to reactions with high yields, purities, and sometimes “greener” processes with significantly reduced reaction times [4-6].
The question in mind has to be: Is that everything or can we go any further? The answer will be given in a short summary presented below and in detail in this book.
Microreactors now introduce the possibility to perform reactions in a confined space, enabling excellent heat transfer and tremendous mass transfer, which could never be met by the established batch chemistry. Keeping the novel process window in mind, it enables completely new processing pathways in chemistry where reactions are possible, which cannot be done in conventional batch/flask systems, such as protecting group-free synthesis and flash chemistry.
Keeping the system at the thermal runaway state can be of great interest for highly exothermic reactions with high activation energies [7]. This can be easily combined with heat pipes: To start the reaction, the pipes are heated to the activation temperature of the respective reaction. After reaching the activation energy, the released heat from the reaction behind the mixing chamber is discharged through the heat pipe and injected back to preheat the educts. An excess heat can be easily dissipated by commercial computer cooling systems [8, 9]. Examples for such reactions are nitration reactions without solvents [10], diazomethane synthesis [11] with subsequent in situ reaction to azo dyes, and oxidations with (singlet) oxygen in a falling film reactor [12]. If passive mixing through energy input from the pumps is not sufficient, active mixing
can also be enabled by installing ultrasonic or acoustic probes [13]. Other possibilities for active mixing include integrated microvalves, electro wetting, and vibrating membranes, which can also be used for introducing substrates over time or exchanging ions [14].
Another great opportunity for the use of microreactors are research fields where high cross-phase surfaces are needed. For instance, heterogeneous catalysis is of great importance for high-tech chemicals such as liquid crystals for LCDs [4]. Coating microreactors with those catalysts enables surface areas comparable to packed bed reactors with palladium on charcoal packing, but with the advantage of easier recycling of the catalyst, which is removed from the reactor surface by dissolution and recoating of the reactor [7].
For developing new products, this knowledge should be used at the earliest possible stage in industrial research. Facilitating up-scaling due to the retention of process parameters is an important byproduct of applying microchemistry and further process design and engineering.
Other factors, such as safety issues and human resource minimization, as well as waste treatment play an important role in life cycle assessments. Safety concerns emerging through the use of high pressure and temperature can be invalidated by energy reflection: The inner volume of a microreactor is usually too small to hold enough substance at one time for an explosion, which could harm people or the building stability. To minimize the personnel needed to operate the facility, automation of these processes is also of great interest. To account for this, a variety of probes monitoring pressure, temperature, and also product quality with in-line high-performance liquid chromatography (HPLC) or near-infrared ((N)-IR) measurements can be installed in the system. Furthermore, the easy installation of computer controlled emergency purges can make the facility almost 100% automated without any need of human intervention. This not only reduces recurring costs but also improves safety due to elimination of human error. These facilities can be built in parts, fitting in a small number of overseas containers, for better portability [5].
Process intensification of already existing batch or continuous processes is not practical in most cases because of substantial investments made for optimizing the parameters. Achieving significantly better space/time-yields or a higher degree of sustainability is not possible by using only standard processing techniques without a total redesigning of the process and reinvestigation of each step. Most of the chemical companies in the European Union are complying with GMP guidelines, which do not allow altering of process parameters and process layouts without external validation of these changes. Combining these presumptions and keeping the cost factor in mind, changing existing and stable running processes in the sense of process intensification can only be of benefit for highly exothermic reactions or systems that rely on excellent mixing quality.
However, there is more potential in this field additionally to novel process windows. All these processes, even the “brand new” ones, still deal with standard heating
techniques using oil/water or standard cooling techniques using aggregates. Both techniques are highly energy consuming and can be easily replaced by incorporating existing temperature control setups from other fields. For instance, heating can be achieved in a very ecological way by using solar mirrors designed for solar power plants. Simple cooling can also be realized using the heat pipes designed for high performance cluster computer systems which have already been mentioned above. This would not only reduce the energy needed to operate the facility but also contribute significantly to the sustainability of the process lowering it to dimensions which could not be achieved with any other technology besides microreactor technology.
Even so, there is one step to go: novel process windows, intensifying mass and heat transfer capabilities, and temperature control is still not “chemistry at the limit”. This should imply driving the reaction at the absolute kinetic limit, eliminating all decelerating elements which were introduced in the early stages of continuous processing, to keep the reaction controllable with limited heat and mass transfer. For instance, those factors are solvents, limiting the reaction through diffusion control, and heat exchange, limiting the product yield by overheating. Substrates could be used without diluting and heat exchange can be driven to the limit by using vapor-driven heat exchangers (heat pipes) in direct contact with the reaction chamber. The last point could be achieved by directly etching the reactor layout into commercially available heat pipes.
Bibliography
- [1] Ehrfeld, W., Hessel, V., Löwe, H.; Microreactors, Wiley-VCH, Weinheim, 2000.
- [2] Hessel, V., Kralisch, D., Kockmann, N., Noel, T., Wang, Q.; “Novel process windows for enabling, accelerating, and uplifting flow chemistry”, ChemSusChem 6, 5 (2013) 746-789.
- [3] Hessel, V., Cortese, B., de Croon, M. H.J.M.; “Novel process windows – concept, proposition and evaluation methodology, and intensified superheated processing”, Chem. Eng. Sci. 66, 7 (2011) 1426–1448.
- [4] Jensen, K. F.; “Microreaction engineering – is small better?” Chem. Engin. Sci. 56 (2001) 293-303.
- [5] Kralisch, D., Streckmann, I., Ott, D., Krtschil, U., Santacesaria, E., Die Serio, M., Russo, V., De Carlo, L., Linhart, W., Christian, E., Cortese, B., de Croon, M. H.J.M., Hessel, V.; “Transfer of the epoxidation of Soybean oil from batch to flow chemistry guided by cost and environmantal issues”, Chem. Sus. Chem. 5 (2012) 300-311.
- [6] Huebschmann, S., Kralisch, D., Löwe, H., Breuch, D., Petersen, J. H., Dietrich, T., Scholz, R.; “Decision support towards green process design in microstructured reactors by accompanying (simplified) life cycle assessment”, Green Chem. 13, 7 (2011) 1694-1707.
- [7] Kressirer, S., Protasova, L. N., de Croon, M. H.J.M., Hessel, V., Kralisch, A.; “Removal and renewal of catalytic coatings from lab- and pilot-scale microreactors, accompanied by life cycle assessment and cost analysis”, Green Chem. 14 (2012) 3034–3046.
- [8] Löwe, H., Axinte, R. D., Breuch, D., Hang, T., Hofmann, C.; “Heat pipe cooled microstructured reactor concept for highly exothermal ionic liquid syntheses”, Chem. Eng. Technol. 33, 7 (2010) 1153-1158.
- [9] Ehm, N., Löwe, H.; “Heat pipe mediated control of fast and highly exothermal reactions”, Org. Proc. Res. Dev. 15, 6 (2011) 1438-1441.
- [10] Hessel, V., Löb, P., Löwe, H.; “Industrial Microreactor Process Development up to Production”, in Wirth, T. (Ed.) Microreactors in Organic Chemistry and Catalysis, pp. 211-275, Wiley-VCH, Weinheim, 2008.
- [11] Mueller, G., Gaup, T.; “Continuous diazomethane chemistry”, Chem. Files, Vol. 5, 25 August (2005), 8-9.
- [12] Jähnisch, K., Dingerdissen, U.; “Photochemical generation and [4+2]-cycloaddition of singlet oxygen in a falling-film microreactor”, Chem. Eng. Technol. 28, 4 (2005) 426-427.
- [13] Yang, Z., Matsumoto, S., Goto, H., Matsumoto, M., Maeda, R.; “Ultrasonic micromixer for microfluidic systems”, Sensor Actuat. A 93 (2001) 266-272.
- [14] Hessel, V., Löwe, H., Schönfeld, F.; “Micromixers – a review on passive and active mixing principles”, Chem. Eng. Sci. 60, 8-9 (2005) 2479-2501.
![]()
Part II: Theoretical foundations
dp n="26" folio="8" ? dp n="27" folio="9" ? ![]()
Ferenc Darvas and Dorman Gyorgy
2 Fundamentals of Flow Chemistry
2.1 Fundamentals of chemical reactions
Objective of this chapter
This chapter intends to summarize all the important basic theoretical features of flow chemistry and flow reactors. The detailed discussion of the particular topics can be found in the following chapters. At each section such chapters are highlighted.
2.1.1 Thermodynamic requirements for reaction
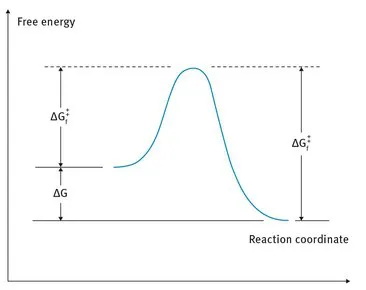
In order for a reaction to take place spontaneously, the Gibbs free energy of the products must be lower than the free energy of the reactants; that is, G must be negative.
ΔG = ΔH – T · ΔS
Free energy is made up of two components, enthalpy H and entropy S.
G: free energy, H: enthalpy, T: temperature, S: entropy, Δ: difference (change between original and product).
Fig. 2.1: Free energy profile of a chemical reaction without having an intermediate.
The ...