
G3P - Good Privacy Protection Practice in Clinical Research
- 210 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
G3P - Good Privacy Protection Practice in Clinical Research
About This Book
Establishing ethical and privacy protection aspects in scientific research, especially in medical research, has a long history. Medical data are usually more sensible than other personal data and require therefore an even higher degree of protection than other personal data. In recent research projects genetic evaluations become more and more important and trigger thereby new and continuing activities in the context of data protection. Genetic data as a subset of medical data are the most sensible category of personal data and require therefore the highest degree of data protection.
The book provides a systematic and itemized approach to data protection in clinical research including the handling of genetic material, genetic samples as well as derived genetic data and the subsequent secure storage of them. The set up of different kinds of clinical trials having in addition a genetic part, the concept of a genetic informed consent as well as collection schemes of samples are described in detail. Technical requirements and aspects of data protection including pseudonymization and anonymization procedures taking into account ethics committees requirements as well as the underlying legal framework are also presented.
Without any exception, all principles and methods presented are best practices, repeatedly applied in different clinical environments and by no means theoretical considerations.
Frequently asked questions
Information
1 Introduction
- (a) Human genetic data have a special status because:
- (i) they can be predictive of genetic predispositions concerning individuals;
- (ii) they may have a significant impact on the family, including offspring, extending over generations, and in some instances on the whole group to which the person concerned belongs;
- (iii) they may contain information the significance of which is not necessarily known at the time of the collection of the biological samples;
- (iv) they may have cultural significance for persons or groups.
- – Agreed Terminology in genetic data processing, i.e. unambiguously define the terms you use.
- – Relevant Legal Requirements, laws, directives, regulations, i.e. be informed about legal requirements and/or boundaries that must be taken into account.
- – Data Protection – protect the privacy of personal data, i.e. develop sensitivity and respective measures to guarantee a necessary and high level of data protection.
- – Data Security – control the access to personal data, i.e. protect the physical security of personal data, adjust data security measures to the actual standards.
- – Informed Consent for the participation in the clinical as well as in the genetic part of a clinical study – agreement which evaluations may be performed.
- – withdrawals from either part of a clinical study, clinical as well as genetic one at any time and if ever possible,
- – and survey the workflow of samples collected for genetic analysis,
- – possible restrictions to genetic analysis required most likely by Ethics Committees resp. IRBs,
- – the coding or anonymization of data and samples,
- – the secure storage of genetic data, original as well as derived data,
- – requests for a common statistical analysis of coded (e.g. anonymized) clinical and related genetic data.
2 Study Modes
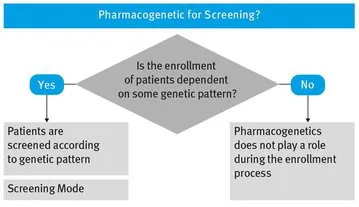
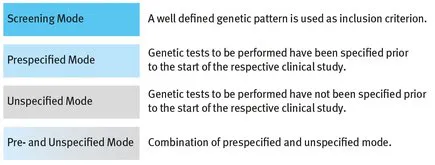
2.1 Screening Mode – Pharmacogenetic Information for Screening
2.2 Pre-Unspecified Mode – Pharmacogenetic as Part of a Study
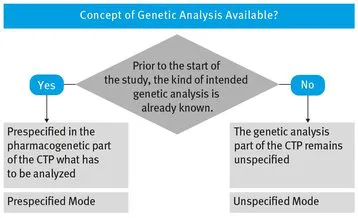
- – We know for certain, prior to the start of the study, which kind of genetic analysis (genetic tests) should be performed. In this case, all genetic tests to be carried out must be prespecified in the Clinical Trial Protocol (CTP). We speak of Prespecified Mode.
- – We do not know for certain, prior to the start of the study, which kind of genetic analysis should be performed. In this situation we cannot specify genetic tests to be carried out in the CTP, i.e. genetic tests to be carried out later remain unspecified at that moment. Therefore, we speak of Unspecified Mode.
- – If you have a concrete idea with respect to genetic analysis, start with prespecified mode, and if you think there could be some more genetic analysis needed in the future, collect additional samples for later analysis (unspecified mode).
- – Prespecified Mode – essentially „business as usual“.
- – Unspecified Mode – anonymization of data and samples is required prior to the start of a genetic analysis (cf. later).
2.3 Possible Approaches, Summary – Clinical Trial With Genetic Part
- – If the genetic part is used as inclusion criteria, i.e. the verification of a specific genetic pattern is needed for the inclusion of a subject, you have to apply Screening Mode (genotyping)
- – If you know in advance (prior to the start of the study), which genetic tests are to be evaluated later, you apply Prespecified Mode
- – If you do not want or cannot specify in advance which genetic tests are to be evaluated later, then samples are collected, the DNA is extracted and stored for later analysis. You have to apply Unspecified Mode
- – If you know in advance (prior to the start of the study), which genetic tests are to be evaluated later, and if you want to collect in addition samples for later analysis, then you have to apply Prespecified Mode on the first stage, andUnspecified Mode on the second stage
- – A trace back to the patient‘s identity is possible, but only the investigator knows the patient‘s identity.
- – Data (clinical as well as genetic data) are de-identified by a code, e.g. PAT_NO (Patient Number).
- – Essentially, samples will be destroyed after reporting of results.
- – Genetic data analysis can be part of a submission....
Table of contents
- Also of Interest
- Title Page
- Authors
- Copyright Page
- Preface
- Table of Contents
- About the Authors
- 1 Introduction
- 2 Study Modes
- 3 Protection Masks and Procedures
- 4 Coding Methods for De-identified Samples/Data
- 5 Relationships Among the Protection Masks
- 6 Data Types
- 7 Anonymization
- 8 Validation – a Brief Introduction
- 9 Request Management
- 10 Legal Requirements & Regulations
- 11 Informed Consent
- 12 Selected Data Protection & Medical Sites
- 13 Impact of External Services on Data Protection
- 14 Practical Approach to Clinical Trials with SupplementaryGenetic Parts
- Appendix
- 20 Abbreviations
- 21 References
- Index