
- 120 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Distillation
About this book
Distillation basedon Mass Transfer Processes, starting from the basic equation of ternary distillation published by Hausen in 1932 and exploiting the properties of this equation covering all modes of distillation. The material is intended as a graduate textbook for an advanced course on distillation but will also help the practicing engineer to better understand the complex interrelationships of multi-component distillation.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1 The principles and modes of distillation
The principle of distillation is based on the thermodynamic property of most liquid mixtures that the vapour produced from a boiling mixture has a composition enriched in the lower boiling components of the liquid which allows to separate a liquid mixture into fractions with compositions different from the liquid mixture and even into its components. The most basic devices of implementing a distillation are the discontinuous or batchwise distillation via the simple distillation or the continuous flash distillation.
1.1 Simple distillation
Figure 1.1 shows a set-up to carry out such a “simple distillation”. It consists of a heated still-pot, a condenser to liquefy the vapour produced in the still-pot and a receiver to collect the distillate. If heat is continuously added to the still-pot, part of the liquid in the still-pot will vaporize and assuming that the components of the liquid mixture have a different vapour pressure, the vapour leaving the still-pot will be enriched in the components with a higher vapour pressure resulting in a distillate in the receiver different from the liquid mixture in the still pot. By evaporating part of the liquid in the still-pot the initial mixture in the still-pot is separated into two fractions, i.e. the residue in the still-pot and the distillate in the receiver. The separation effect will be small, however, unless the compositions of the liquid and the vapour produced from the liquid are substantially different.
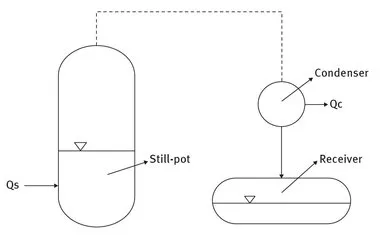
Fig. 1.1. Simple-Distillation.
1.2 Flash distillation
In a flash distillation according to Fig. 1.2 the mixture to be separated (Feed) is fed continuously to the flash-drum and with the addition of heat (Qs) separated into the two fractions distillate and bottom product. The amount of the two fractions depends on the amount of heat added and the separation effect is, like in simple distillation, given by the thermodynamic properties of the feed. Again, the separation effect will be small unless the vapour pressure of the components differs substantially.
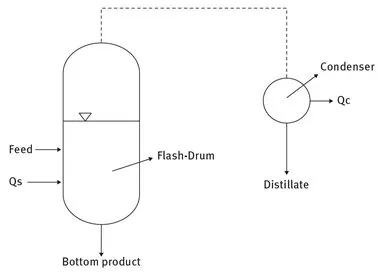
Fig. 1.2. Flash-Distillation.
1.3 Multistage distillation
In the simple as well as the flash distillation it is not possible to obtain one of the components as an almost pure component since the original mixture is separated into two fractions with all components present but at a composition different from the original mixture. Only by a connection in series of the simple distillation or the flash distillation in form of a cascade, the so-called rectification or multistage separation process as shown in Fig. 1.3, is it possible to separate a mixture into fractions with lesser components as the original mixture or even into almost pure components. Since most technical distillations are operated in the continuous mode, the batchwise distillation will not be discussed further.
In practice the cascade is realized in form of a tower or column with the addition of the feed at an optimized location and a counter current flow of the liquid and the vapour within the rectifying section above and the stripping section below the feed location. At the top of the column the distillate is withdrawn either as a vapour (not
shown in Fig. 1.3) or as part of the condensed vapour with the rest of the condensate returned to the column in order to provide for the liquid down-flow in the column. The liquid withdrawn from the bottom of the column is divided into the bottom product with the rest vaporized and returned to the column providing the necessary vapour upflow. The internals of the column consist either of horizontal stages approximating the connection in series of the flash-drums of the cascade as shown by the column to the left in Fig. 1.3 or the column sections are filled with a packing like Raschig rings e.g. as illustrated by the column to the right in Fig. 1.3 [17]. The internals serve to increase the residence time of the liquid in the column, to provide for a large interfacial area between the liquid and the vapour flow and to enhance the mass transfer between the flows in the column.
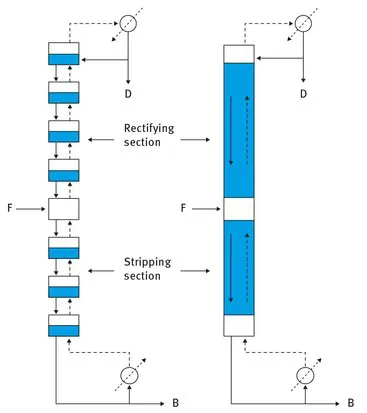
Fig. 1.3. Distillation cascade (– liquid flow - - vapour flow, F = Feed, D = Distillate, B = Bottom product).
Since the vapour rising from a boiling liquid is always enriched in the lower boiling components of the liquid, the composition of the lower boiling components of the vapour flowing upwards in the column increases from the bottom to the top of the column. Thus, starting from a given composition at the feed location, the composition of the vapour in the section above the feed location will be enriched in the lower boiling
components whereas the liquid flowing downwards in the section below the feed location will be stripped of the lower boiling components. The section of the column above the feed location is called the rectifying section, therefore, and the section below the feed location is the stripping section of a column.
Due to this separation effect the composition of the distillate and the bottom product differ from the composition of the feed. With a sufficient length of the respective section it is possible to obtain an almost pure component or to split the feed into two fractions with the components of one fraction not present in the other fraction. Taking for instance a feed with three components A, B, C and a column with a sufficient length of the rectifying and the stripping section, it is possible to produce the fractions A/BC, A/ABC; AB/BC, AB/ABC, ABC/C, ABC/BC, ABC/ABC and AB/C whereas a split AC/B is impossible. It follows that a separation of a mixture of n components into almost pure single components requires at least (n − 1) columns. As the possible splits increase exponentially with the number of components, determination of the optimal sequence taking into account constraints like minimum investment and operating costs can become a rather formidable optimisation problem [18].
2 Assumptions and problem reduction
- Ideal mixtures are defined as mixtures with constant relative volatilities.
- The flow rates of the liquid and the vapour in the rectifying and stripping section of a distillation column are considered constant.
- The enthalpy of the liquid and the vapour are considered constant. This assumption does not affect the principle behaviour of a distillation process. The effect of strongly different heats of vaporisation of the pure components of a mixture on the flow rates can be accounted for by using caloric fractions rather than mole fractions [19], [36].
- Except for the simple distillation only continuous, steady-state distillation modes at a countercurrent flow of the liquid and the vapour phase are discussed.
- Any continuous distillation plant consists in principle of mass transfer sections with a countercurrent flow of liquid and vapour which are separated or supplemented by feed and product sections as shown in Fig. 2.1. The basic equations of di...
Table of contents
- Also of Interest
- Title Page
- Copyright Page
- Preface
- Table of Contents
- Introduction
- 1 The principles and modes of distillation
- 2 Assumptions and problem reduction
- 3 The basic equations of distillation
- 4 Distillation of ideal mixtures
- 5 Distillation of real mixtures
- 6 Computer programs
- References
- 7 Nomenclature
- 8 Glossary
- A Appendices
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Distillation by Alfons Vogelpohl in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Industrial & Technical Chemistry. We have over one million books available in our catalogue for you to explore.