![]()
1 The chemical industry
1.1 Introduction
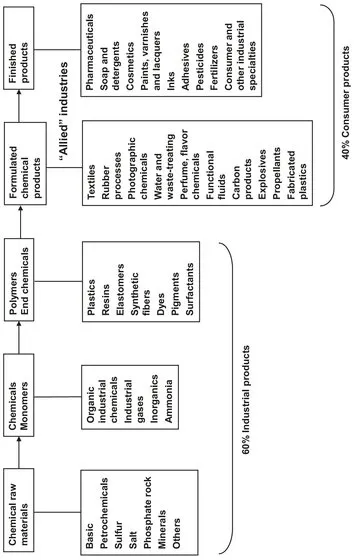
The industry that applies the knowledge of chemical behavior is generally called the chemical process industry. Chemical reactions and separation of compounds are used to obtain products with desired properties. In reality the chemical industry is a set of related industries with many diverse functions and products. Some of these different areas, divided into three general classes of products, are listed in Fig. 1.1:
- (1) industrial chemicals and monomers such as acids, alkalis, salts, chlorine, ammonia, ethylene, propylene, caprolactam, acrylonitrile, industrial gases and other organic chemicals;
- (2) polymers and end chemicals to be used in further manufacture, such as synthetic resins, plastics, fibers, elastomers, dyes and pigments;
- (3) finished chemical products for consumer applications, such as architectural paints, drugs, cosmetics, and soaps, or to be used as materials or supplies in other industries, such as industrial paints, adhesives, fertilizers, and explosives.
Fig. 1.1 emphasizes that certain raw materials are used to prepare key chemicals, monomers and intermediates that may be sold independently or used directly in additional steps to give various polymers and end chemicals. These in turn can be formulated and fabricated into chemical products, which can sometimes be modified into finished products. Hence the term chemicals and allied products accurately represents this diversity as well as the flow of materials and products from raw sources to finished formulations. Although the division is approximate, about 60% of the chemical industry manufactures industrial products that are further modified, whereas 40 % of their products are sold directly to the consumer. Clearly the chemical industry is part of the manufacturing industry, and within this it plays a central part, even though it is by no means the largest part of the manufacturing sector. Its key position arises from the fact that almost all the other parts of the manufacturing sector utilize its products.
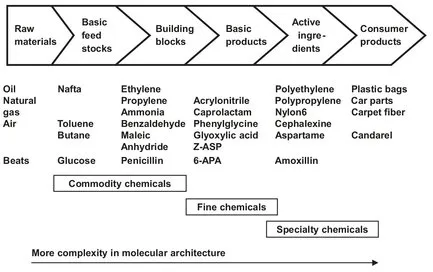
The three major segments of the chemical industry depicted in Fig. 1.2 are related to commodities, fine chemicals, and specialty chemicals. The substances representing these segments exhibit an increasing complexity in molecular architecture. In the business sector, starting from fossil fuels and culminating in the application products, fine chemicals take a position with their special characteristics between the commodities or base chemicals such as toluene, acetic acid, acetone, methanol, etc. and specialty chemicals or desired products for various markets. Fine chemicals are sold on the basis of their chemical composition for use as intermediates in the production of other materials. They are often needed in relatively small quantities and high purity for only one or a few end uses. Product applications can be found in pharmaceutical (50 %), agrochemicals (20 %) flavors and fragrances (5 %), food additives (5 %), and various other industries (20 %). Specialty chemicals are purchased because of their effect rather than composition. There is some overlap in the definitions of fine and specialty chemicals. The European market in fine chemicals is estimated to be approximately € 50 billion in sales, compared to € 500 billion for the commodity segment. With respect to the financial turnover (sales) the specialty chemicals industry is as large as the bulk chemical industry.
Fig. 1.1: Chemical process industry.
Fig. 1.2: Business sector segmentation.
1.2 General characteristics of the chemical industry segments
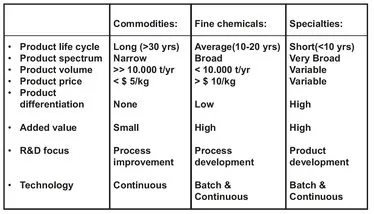
The major sectors of the chemical industry are categorized on the basis of end product uses in Fig. 1.3. Going from commodities, through fine chemicals, to specialty chemicals, the products become higher-priced, have a higher added value, a lower volume, and generally a shorter lifecycle than the products closer to the fossil fuels in the value chain. Within the chemical process industries, batch processing is focused on the fine and specialty chemicals sectors, while continuous processing is dominant in bulk chemicals production. The scale of operations ranges from quite small plants (a few tons per year) in the fine chemical area to the giants (100-1000 thousand tons per year) in the petrochemical sector. Today a typical base petrochemical plant is designed to produce enormous quantities (100-600 ktons/year) of a single product and operate 24 hours a day all year round. They are used to make key intermediates, which are turned into a very wide range of products by further processing. Clearly such large and sophisticated plants require a very high capital investment and are characterized by high investment versus low labor components in the cost of manufacture. The investment per worker in a base petrochemicals olefins plant may well exceed a quarter of a million dollars. Although these plants take full advantage of the economy of scale effect, the losses due to running under design capacity can be extremely high if the balance between production capacity and market demand is disturbed. This is particularly evident when the economy is depressed, and the chemical industry’s business tends to follow the cyclical pattern of the economy, with periods of full activity followed by those of very low activity.
Fig. 1.3: The three main segments of the chemical industry.
It is important to keep in mind that, although most of the discussion about the chemical industry tends to revolve around the multinational giants, the industry is very diverse and includes many small-sized companies as well. There is a similar diversity in the sizes of chemical plants. Batch-type plants are used for the manufacture of relatively small amounts of fine and specialty chemicals, typically up to 100 tons per annum. They are therefore not dedicated to producing just a single product, but are multipurpose and may be used to produce a number of different chemicals each year. In this part of the chemical industry the investment level may not exceed the order of $25 000 per worker.
The research and development carried out in industry can be divided into product development, process development, process improvement, and application development. The nature of the research and development being carried out varies significantly across the various sectors of the chemical industry. In the commodity chemicals sector, most of the R & D expenditure will be devoted to process improvement. Process improvement relates to processes which are already operating. It may be due to problems which have arisen which have hindered or stopped production. More commonly, however, process improvement will be directed at improving the profitability of the process. Improving the quality of the product, by process modification, may lead to new markets for the product. In recent years the most important process improvement activity has been to reduce the environmental impact of the processes. At the other end of the scale lie the specialty chemicals. Here there are immense and continuous efforts undertaken to discover and develop new products which exert the desired, specific effect. As such, the main focus in this sector is on new product development, such as pharmaceuticals, agrochemicals, and antioxidant additives. Process development absorbs considerable resources in the fine chemicals industry, in part because of the shorter life cycles of fine chemicals as compared to commodities. It covers developing new manufacturing processes for new as well as existing products. The push for the latter may originate from the availability of new technology or a change in the availability and/or cost of raw materials. Process development for a new product depends on things such as the scale on which it is to be manufactured, the by-products formed, and the required purity.
1.3 Major raw materials
Inorganic chemicals are derived from many different sources. In contrast the production of organic chemicals is almost entirely based on two raw material sources: crude oil and natural gas. At present more than 90 % (by tonnage) of all commercially important organic chemicals are produced from crude oil and natural gas via a multitude of petrochemical processes. Compared to crude oil, natural gas is less versatile, because carbon-carbon bonds have to be built up. Apart from crude oil and natural gas, other raw materials are used on a smaller scale, such as fatty oils, starch, sugar and molasses, wood, and straw.
The inorganic chemical industry is based on a large variety of minerals, air, and water. Minerals are converted into products like building materials and pigments. There is, however, a group of raw materials from which a limited number of rather important inorganic intermediates is made. Perhaps the most notable example is sulfur. Substantial quantities of sulfur are also removed and recovered from natural gas and crude oil. Over 80 % of all sulfur is converted into sulfuric acid,...