
- 299 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Hydrochemistry
About this book
Water is the basis of all life. Preservation of aquatic ecosystems and protection of water resources thus are among the most important goals of a sustainable development. The quality of water is mainly determined by its constituents, the entirety of the substances dissolved or suspended in water. To assess the water quality on a sound basis requires in-depth knowledge about the occurrence, behavior and fate of these constituents. That explains the importance of hydrochemistry (also referred to as water chemistry or aquatic chemistry) as a scientific discipline that deals with water constituents and their reactions within the natural water cycle and within the cycle of water use.
This textbook introduces the elementary basics of hydrochemistry with special focus on reaction equilibria in aquatic systems and their mathematical description. It is designed as an introductory textbook for students of all environment-related courses who are beginning their hydrochemical education. Only minor knowledge in General Chemistry is required to understand the text. The book is also suitable for continuing education.
Topics discussed in this textbook include: structure and properties of water, concentration measures and activities, colligative properties, basics of chemical equilibria, gas-water partitioning, acid/base reactions, precipitation/dissolution, calco-carbonic equilibrium, redox reactions, complex formation, and sorption. The text is supplemented by numerous figures and tables. More than 50 examples within the text as well as more than 60 problems to be solved by the reader support the acquiring of knowledge. Complete and detailed solutions to all problems are given in a separate chapter.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1 Introduction
| Water resource | Volume (103 km3) | Portion (%) |
|---|---|---|
| Oceans | 1 335 040 | 96.95 |
| Polar ice caps and glaciers | 26 350 | 1.91 |
| Groundwater | 15 300 | 1.11 |
| Lakes and rivers | 178 | 0.013 |
| Soil moisture | 122 | 0.009 |
| Permafrost | 22 | 0.0016 |
| Atmosphere | 12.7 | 0.0009 |
| Total | 1 377 024.7 | 100.0 |
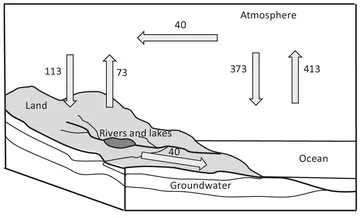
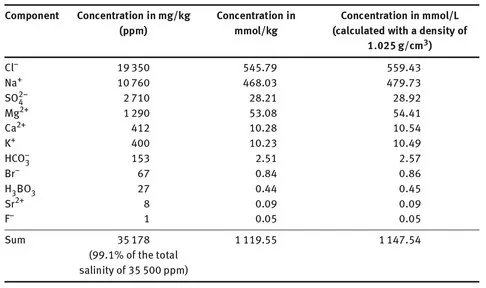
Table of contents
- Titel
- Impressum
- Preface
- Inhaltsverzeichnis
- 1 Introduction
- 2 Structure and properties of water
- 3 Concentrations and activities
- 4 Colligative properties
- 5 The chemical equilibrium: Some general aspects
- 6 Gas-water partitioning
- 7 Acid/base equilibria
- 8 Precipitation/dissolution equilibria
- 9 Calco-carbonic equilibrium
- 10 Redox equilibria
- 11 Complex formation
- 12 Sorption
- 13 Solutions to the problems
- A Appendix
- Nomenclature
- Bibliography
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app