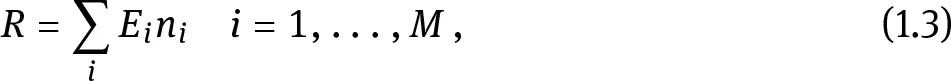![]()
Alberto Del Guerra and Daniele Panetta
1Radiation measurement
Aim: The interactions of radiation with matter are the basic processes underlying the working principle of every radiation detection apparatus. This chapter focuses on the methods and techniques for the detection of ionizing radiation. First, the different types of ionizing radiations (relevant in the context of radiochemistry) will be introduced. In order to understand how a certain radiation can be qualitatively and/or quantitatively assessed, it is important to figure out how it interacts with the matter it traverses. In most cases, the result of such interaction is measured as an electric signal by means of dedicated electronics, and then displayed on some suitable screen or printer. Such “active” detectors allow a real-time measurement. Other detectors do not provide a direct reading, as they have to be exposed to the radiation field for a given period of time: these detectors are called “passive”. Another way to distinguish among the various types of detectors is based on the physical and chemical properties of the substance that constitutes the sensitive volume of the detector itself. They can be gaseous, liquid or solid. The choice of one or another type of detector is mainly based on its final purpose: some are suitable as portable survey meters, others are better suited for personal monitoring, or for probing the radiochemical purity of a radiopharmaceutical or detecting it for the purpose of molecular imaging.
1.1Physical basis of radiation detection
1.1.1Types of radiation
The term radiation is applicable to every physical entity that is able to transport energy through space and time, away from the place where it originated (commonly referred to as the source of the radiation). In the present context, radiation is emitted in the course of transformations of unstable nuclei and interacts with matter. This interaction is at the basis of radiation measurement. Several types of radiation can be distinguished.1 In the field of radiochemistry and nuclear chemistry we will only deal with electromagnetic radiation and charged particles that can ionize the matter they interact with. For this reason, these types of radiation are called ionizing radiation. Table 1.1 classifies the relevant types.
Tab. 1.1: General classification scheme for radiation based on its ability to ionize matter.
| Ionizing | | Nonionizing |
| directly ionizing (electrically charged) | indirectly ionizing (electrically neutral) | |
| α, β+/β− and atomic electrons, p, δ and fissions fragments | X, γ, hard UV, and n | electromagnetic waves and particles with < 10 eV energy (e.g., infrared radiation, visible light, soft UV) |
1.1.2Definitions
In this context “ionizing radiation” refers to radiation emitted from the primary (such as beta electrons, α-particles, neutrons and fission fragments), secondary or posteffect (such as internal conversion electrons or AUGER and COSTER–KRONIG electrons, γ and X-ray photons and bremsstrahlung) transformations of an unstable nuclide. The source might have various dimensions, ranging from point-like or monoatomic layers to volumes of milliliters, liters or (in industrial processes) even cubic meters, to solids of various chemical and physical state and size, and to gases. The radiation emitted from a source distributes in all directions (4π geometry). Thus it creates a radiation field. A radiation detector represents a well-defined state of condensed matter, which translates the effect of radiation interaction in that particular matter into a quantitative and qualitative signal.
The energy transported by ionizing radiation is either represented electromagnetically or associated to the kinetic energy of fast nuclear or elementary particles after they have originated from a source. All particles with an electric charge can directly ionize atoms when they interact with matter through multiple collisions driven by coulomb forces: they are called directly ionizing radiation, cf. Vol. I, Chapter 12. Examples of directly ionizing radiation are α-particles, protons, light or heavy ions, muons and other elementary particles with sufficient kinetic energy. Neutral particles and electromagnetic waves (e.g. photons) do not have electric charge; they can ionize atoms through few, sparse interactions. These latter interactions give rise to secondary charged particles that, in turn, can continue to ionize the surrounding atoms. Secondary electrons with sufficiently high recoil kinetic energy to travel into the matter producing further ionization on atoms that are far away from the site of interaction with primary radiation are called delta rays. Thus, neutral particles can ionize the matter indirectly: for this reason this is called indirectly ionizing radiation. Commons examples of indirectly ionizing radiation are neutrons, X-rays and γ-photons.2
1.1.3Parameters describing a radiation field
In order to allow a quantitative description of radiation fields, some basic definitions could be useful in the following sections. One could think of a radiation field as a portion of three-dimensional space in which several particles move along individual trajectories. At a given point P in the field there is a particle fluence (or flux), Φ, defined as the number N of particles passing in a time interval Δt through a sphere of crosssectional area s centered in P, when s becomes infinitely small. More formally:
Because Φhas the dimension of the inverse of an area, it is measured in m−2. Of course, the number N is a stochastic quantity; thus, it is intended in the above equation that the expectation value of N must be considered for the computation of Φ in a sufficiently long time interval.
The time derivative of eq. (1.1) gives another quantity expressing the number of particles passing through P in the unit time. It is called fluence rate (or flux density) and is denoted by ϕ:
The unit of measureof the particle fluence rate is m−2 s−1. The fluence and fluence rate, as described above, only provide information about the number of particles crossing a certain portion of space. Sometimes it is useful to have information not only about the number of particles, but also about the total energy transported by them. Let us denote by R the total kinetic energy of the N particles through P:
where ni is the number of particles having energy E i, M is the total number of energies available in the field, and Σi ni = N is the total number of particles. The distribution of particles ni in each energy level in the field is called the energy spectrum of the radiation. For simplicity, eq. (1.3) supposes that only a finite number of energies are present among all the N particles: in this case there is a discrete energy spectrum. Examples of radiation fields with a discrete spectrum are the photon field originating from a γ-emitting radionuclide (e.g., 99mTc, widely used in nuclear medicine, with main gamma emission of 140.51 keV) or the particle field originating from an α-emitter (such as 210Po, with Eα = 5.3 MeV, often used in polonium-beryllium alloys to create neutron sources). In the most general case, the energy of the particles in a field can have an arbitrary value in a continuous interval between 0 and Emax. In this case, the summation in eq. (1.3) is replaced by an integral, and the radiation field is referred to as a field with a continuous energy spectrum. Examples of radiation fields with continuous spectra are the β-electron field emitted by e.g. 14C, the positron field emitted by e.g. 11C (widely use...