
eBook - ePub
New-Generation Bioinorganic Complexes
- 188 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
New-Generation Bioinorganic Complexes
About this book
Bio-Inorganic compounds are successfully applied as therapeutic agents since decades. Thus, scientist designed new metal complexes bearing biomolecules as ligands, investigating their potential as bioactive and therapeutic agents. This book presents a comprehensive overview on materials design, substance classes and their characterization. This book is compiled for scientists interested in medical application of bioinspired materials.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Osamu Yamauchi
1Noncovalent interactions in biocomplexes
1.1Introduction
Noncovalent interactions perform essential roles in biological systems such as molecular recognition, protein stabilisation, and specificity and efficiency of enzymatic reactions [1–4]. They are formed and cleaved instantaneously and are dependent on such factors as the properties of the interacting groups or atoms, the distances between them, and the media in which they are present. The interactions, which are often called weak interactions, are also important for metal complex systems involving nucleotides and DNA [5, 6] and supramolecular architecture [7, 8].
Essential transition metal ions such as copper and zinc are bound to proteins mainly by the side chain groups of the amino acid residues such as the histidine (His) imidazole, cysteine (Cys) thiol, and tyrosine (Tyr) phenol moieties. The ligands forming the metal site may be in contact with the amino acid residues forming the molecular environment through weak interactions, and therefore the metal centre is under conditions which are different from bulk water. Such interactions protect the metal centre from the attack of solvent molecules and have subtle effects on the properties of the metal ion. The function of metalloproteins therefore depends on the active site structure and the noncovalent interactions with the molecular environment. As seen in cytochrome c peroxidase [9, 10] and type 1 copper sites [11], interactions between the coordinating groups such as the imidazole and thiolate moieties and the protein side chain groups surrounding the metal site can influence the structure and electron density and thus the reactivity of the metal centre.
However, various metal ions and complexes are known to be enzyme inhibitors [12], and new steps toward metallodrugs [13–16] and functional complexes considering the second coordination sphere [17] have been made, which indicate that noncovalent interactions and structural fitness are important for the activity.
DNA is well known as the target of the anticancer drugs such as cisplatin and its analogues and metallo-intercalators, the latter of which bind with DNA by noncovalent interactions, especially aromatic ring stacking and electrostatic interactions. Studies have been carried out for developing effective and specific metallo-intercalators and metallo-insertors and clarifying their binding modes [5, 18]. Zinc finger proteins are a class of proteins that bind with Zn(II) to form finger structures with basic, polar, and aromatic amino acids such as arginine (Arg) and His at the finger domains, whose noncovalent interactions with DNA have attracted much attention [19, 20].
In view of the importance of noncovalent interactions in biological systems, this chapter is intended to give a perspective of noncovalent interactions in and around the metal centre and their relevance to the metal site of proteins, focusing on ligand‒ligand interactions in metal‒amino acid and related complexes and interactions involving metal complexes and proteins.
1.2Noncovalent interactions in metal complexes
In the past 50 years there has been growing interest in noncovalent interactions in metal complexes of biological ligands. This section will give an overview of the backgrounds and basic findings related to metal‒ligand systems.
1.2.1Some historical backgrounds
An early indication of intramolecular ligand–ligand interactions was provided by the solution studies on ternary (mixed ligand) complexes of Cu(II) etc. Preferential formation of ternary complexes depending on certain combinations of ligands has been shown by Sigel and his collaborators by evaluation of the stability enhancement relative to the complexes without such a ligand set [21–23]. Studies have been reported for the intramolecular ligand‒ligand stacking interactions in ternary Cu(II) complexes with aromatic diimines (DA) and nucleotides such as Adenosine 5'-monophosphate (AMP) [22, 24–26], and complexes containing amino acids with aromatic, aliphatic, charged, or polar side chains capable of various interactions have been studied [27–29].
Metal transport in biological systems has been an important and interesting subject from the view point of bioinorganic chemistry. Most of the Cu ions (ca. 95 %) in blood serum are bound to ceruloplasmin and are not exchangeable, and the rest are present mainly as Cu(II)‒serum albumin and to a smaller extent as mixed amino acid complexes containing His, both of which are considered to be involved in copper transport [30, 31]. A ternary complex containing His and threonine (Thr), Cu(His)(Thr), was detected in human blood serum [32], while the tracer studies using 64Cu showed that the amino acids Thr, glutamine (Gln), and asparagine (Asn) effectively formed ternary complexes with His, Cu(His)(L) (L = Thr, Gln, and Asn) [33]. The structure of Cu(His)(Thr) was established by X-ray analysis to have His bound to Cu(II) through the amine and imidazole nitrogens with the carboxylate oxygen at an axial position [34]. Later the structure of Cu(His)(Asn) (Fig. 1.1(a)) was revealed to have the same coordination structure as that of Cu(His)(Thr) [35].
Preferential formation of certain ternary amino acid Cu(II) complexes was also indicated by computer simulation of Cu(II)-amino acid systems in solution. Some discrepancies between the tracer experiment and computer simulation regarding the preferred formation of the above mentioned mixed amino acid complexes have been carefully reinvestigated, and the conclusions from both approaches are now in satisfactory agreement [36].
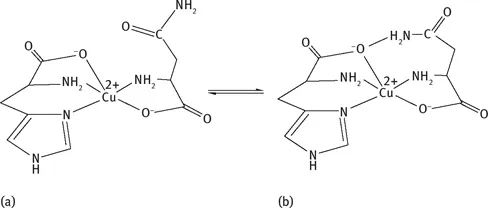
Fig. 1.1: Structures of Cu(His)(Asn) (a) and its possible conformational isomer with ligand‒ligand interaction (b) [35, 37].
Since the side chains of all the above amino acids L have a polar group, the structure of Cu(His)(Asn) suggested the possibility of hydrogen bondin...
Table of contents
- Cover
- Title Page
- Copyright
- Preface
- Contents
- List of contributing authors
- 1 Noncovalent interactions in biocomplexes
- 2 Photo-sensitive complexes based on azobenzene
- 3 Complexes of biogenic amines in their role in living systems
- 4 Synthetic aspects, crystal structures and biological activities of d- and f-metal salen-type complexes
- 5 Biocomplexes in radiochemistry
- 6 Peptides and biocomplexes in anticancer therapy
- 7 Developments in platinum anticancer drug
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access New-Generation Bioinorganic Complexes by Renata Jastrzab, Bartosz Tylkowski, Renata Jastrzab,Bartosz Tylkowski in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Biochemistry. We have over one million books available in our catalogue for you to explore.