![]()
CHAPTER 1
Natural Products in Fruit and Vegetables
1.1 INTRODUCTION
The constituents of edible plants have been the subject of investigation throughout the development of chemistry. Many of the major carbohydrates, fatty acids, amino acids, mineral and vitamin constituents of fruits and vegetables together with their pigments and flavours were isolated and identified during the nineteenth and the first half of the twentieth centuries. In the second half of the twentieth century the advent of instrumental methods of separation and analysis, such as gas chromatography linked to mass spectrometry, high pressure liquid chromatography, ultra-violet, infra-red and nuclear magnetic resonance spectroscopy, permitted far more detailed investigations. Furthermore, the realization that particular components of fruit and vegetables conferred specific health benefits provided the stimulus for more extensive investigations to identify the bioactive natural products.
The organic compounds that occur in plants fall into three main groups. Firstly, there are the high molecular weight polymeric materials, such as cellulose, starch and lignin, which, together with various proteins and nucleic acids, form the structural, storage, enzymatic and genetic components of the cell. Secondly, there are those compounds of lower molecular weight that occur in the majority of plant cells and which play a central role in the metabolism and reproduction of the cell. These are sometimes known as the ‘primary metabolites’. They include the common sugars, some carboxylic acids and the amino acids that are the constituents of peptides and proteins. There are also heterocyclic compounds that are co-enzymes and others which form part of the nucleic acids. Related to these are the plant hormones and signalling compounds which regulate the overall growth and development of the plant. The third group of naturally-occurring compounds also includes relatively low molecular weight compounds, are those which are characteristic of a limited range of species. These compounds may have insect attractant or deterrant roles, or they may provide a defense against microbial attack. They serve to establish an ecological niche for the plant. These natural products can behave as ‘semiochemicals’ which convey a chemical message between species. In plants, many of these compounds are defensive ‘allomones’ being produced to benefit the source but to the detriment of the receiver, typically an insect herbivore. These natural products are sometimes known as ‘secondary metabolites’ and include many of the compounds that are responsible for the particular health benefits of specific fruits or vegetables as well as their colour and flavour. In this context the organoleptic and beneficial properties of fruits and vegetables are often the summation of contributions from many compounds. The naturally occurring compounds in foodstuffs that are beneficial to man are sometimes called ‘nutraceuticals’. Although these primary and secondary metabolites make significant contributions to plant biochemistry and ecology, they are often present in very small concentrations in the plant, typically milligrams per kilogram of fresh weight.
Whereas many of the primary metabolites exert their biological effect within the cell in which they are produced, the secondary metabolites often exert their biological effects on other cells or species. However, this division between primary and secondary metabolites, whilst useful, is not rigid. Acids derived from primary metabolism form esters with secondary metabolites, amino acids that are constituents of proteins are also the progenitors of the alkaloids and glucosinolates that are secondary metabolites, whilst the common sugars are also found as components of the glycosides of secondary metabolites. The distinction is also blurred in the context of the vitamins and hormones. The former, such as vitamin C, are essential dietary factors for man but are commonly formed by plants. Different plants may produce different but structurally related hormones and use them for similar purposes.
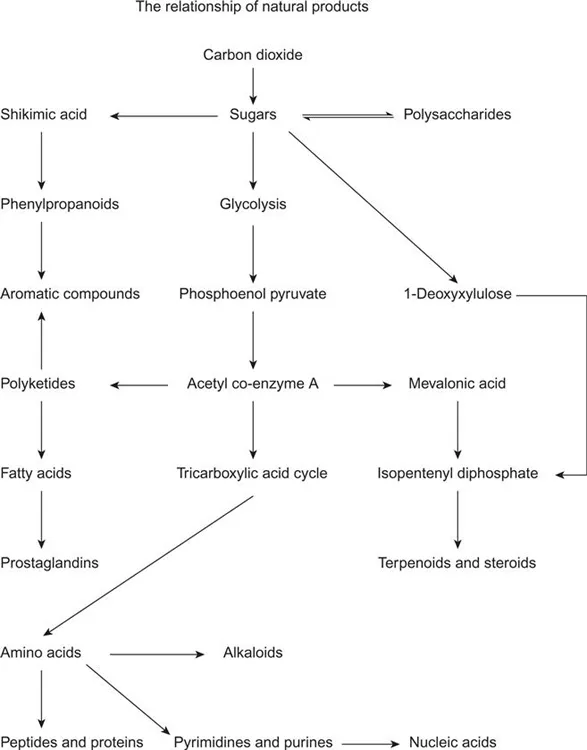
The structures of the secondary metabolites not only vary between species but may also show infra-species variation. Cultivars may be bred for different purposes such as disease resistance, colour, size or taste, all factors which may be determined by their secondary metabolite content. Furthermore, the chemistry of a plant changes as it develops and approaches maturity, as exemplified by the colour and taste of many fruit as they ripen. Some secondary metabolites are also produced as a consequence of external pressures, such as attack by a fungus or herbivore. These variations lead to what may seem at first sight to be a bewildering array of natural products. However, the structural diversity of the natural products that are found in fruit and vegetables can be rationalized in terms of their biosynthesis. A map illustrating these relationships and following the pathway of carbon from carbon dioxide is shown in Figure 1.1. It is helpful to use this scheme as a framework against which to describe the various groups of natural product.
Figure 1.1
1.2 THE BIOSYNTHETIC RELATIONSHIP OF NATURAL PRODUCTS
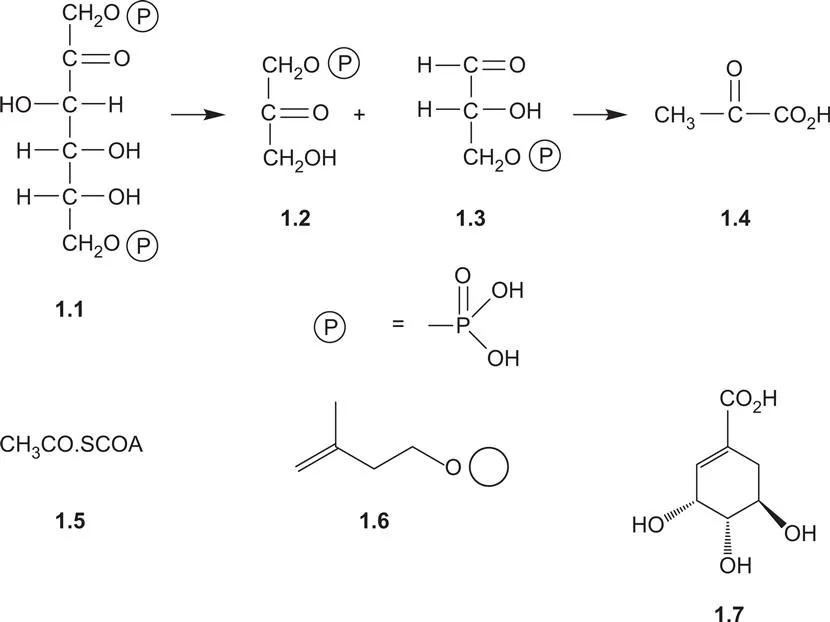
Carbon dioxide from the atmosphere is incorporated by photosynthesis into sugars from which the plant derives some of its structural and storage materials, such as cellulose and starch. Compounds of lower molecular weight may be derived from the sugars. Thus the breakdown of the C6 sugar, fructose 1,6-diphosphate 1.1, by glycolysis affords two C3 fragments, dihydroxyacetone monophosphate 1.2 and glyceraldehyde monophosphate 1.3, through the biochemical equivalent of a retro-aldol reaction. The C3 units provide the source of pyruvic acid 1.4 and thence the acetate units from which many natural products are formed. Acetyl co-enzyme A 1.5 also enters the tricarboxylic acid cycle from which the common plant acids, citric and malic acids, are formed.
Although there are a very large number of natural products, their carbon skeleta are assembled from relatively few elementary building blocks such as acetyl co-enzyme A 1.5, isopentenyl diphosphate 1.6 and shikimic acid 1.7. Pathways involving transamination lead to the formation of amino acids from keto acids and the inclusion of nitrogen into other compounds. The various natural products are then formed by biosynthetic pathways in which these building blocks are linked together, cyclized and oxidized. In many plants it is possible to identify the groups of natural products which they contain, and place these in sequences which reflect their various biosynthetic pathways.
The biosynthetic rationalization of structures not only inter-relates natural products but also reflects the botanical relationships between plants. The occurrence of a particular biosynthetic pathway in a plant indicates the presence of particular enzymes which catalyze steps in the pathway and these in turn reflect the genetic make-up of the plant. Not surprisingly, therefore, botanically related plants produce similar natural products. As we will see in subsequent chapters, the plant families to which various fruits and vegetables belong are often characterized by the presence of particular types of natural product.
1.3 SUGARS
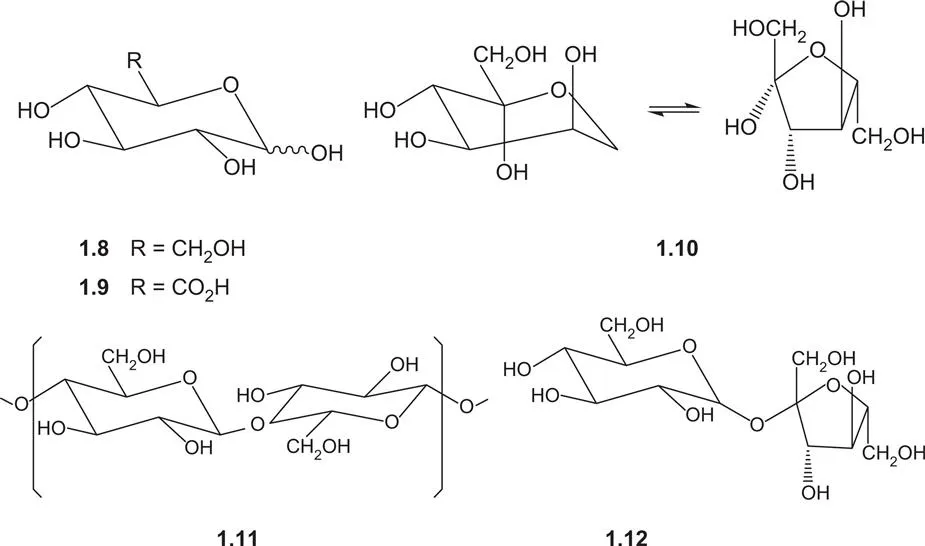
The sugars provide the basic building blocks for the polysaccharide structural materials of fruits and vegetables. The two most widely occurring C6 monosaccharides are the aldose, glucose 1.8, and the ketose, fructose 1.10. The C5 sugars, ribose and deoxyribose, are found in the nucleic acids. Other sugars may be found in glycosides. The majority of naturally-occurring sugars are all related to one enantiomeric form (the D-form) of glyceraldehyde.
The sugars exist in their cyclic hemi-acetal form. In the case of glucose 1.8 this is normally the six-membered pyranose ring, in which all of the hydroxyl groups have the equatorial conformation. Fructose 1.10 is also found in a five-membered furanose form. Another important property of the sugars is their ability to form ether linkages between the hemi-acetal hydroxyl group of one monosaccharide and a hydroxyl group of a second sugar to give a di- and eventually a polysaccharide. Cellobiose 1.11 is a disaccharide derived from a β-l,4 bond between two glucose molecules and which forms the repeating unit of cellulose. Sucrose 1.12, on the other hand, has an α-1,2 link between a glucose and the furanose form of fructose. The ether link between the two hemi-acetal carbons of glucose and fructose masks a potentially reactive centre in each of the monosaccharides restricting some of the reactivity of sucrose and contributing to its role as a useful biological energy storage compound. Maltose has an α-1,4 linkage between two glucose units. In the context of foodstuffs, the importance of the nature of these linkages lies in the variation with which the different glycosidic linkages undergo enzymatic cleavage in man, thus affecting our ability to digest foods.
1.4 STRUCTURAL AND STORAGE POLYSACCHARIDES
Cellulose is one of the most abundant bioorganic polymers. It is a β-1,4′-polyacetal of the disaccharide cellobiose 1.11. The number of sugar units in the polymer may be as high as 10–15000. Due to the many opportunities for both intra- and inter-molecular hydrogen-bonding between the sugar units and the summation of the energy that this represents, a network is created that hinders free rotation and imposes rigidity on the structure. Since the bonding between the sugars involves equatorial rather than axial hydroxyl groups, the polysaccharide adopts an extended linear conformation to give fibrous structures. This provides the basis for the function of cellulose as a structural component of leaves and other parts of the plant.
Although the polymeric structure of cellulose precludes water solubility, the extensive hydrogen-bonding network and the presence of additional sites for hydrogen-bonding mean cellulose can absorb a significant amount of water. This interaction with water confers both advantages and disadvantages to the plant. Since the cellulose in leaves can absorb water, the surface of the leaf is often covered with a hydrophobic hydrocarbon wax to reduce waterlogging when it rains. This wax coating may also prevent excessive water loss from the plant under arid conditions. The development of cultivars with this wax protection to the leaf may be important if we enter a period of long dry summers through global warming.
This wax also behaves as a lubricant and in the autumn it makes freshly fallen wet leaves slippery. When the leaf has died, biodegradation of the wax eventually exposes the cellulose of the leaf which can then become waterlogged and subject to microbial attack. The wax coating on fruit is an important protection against dehydration and microbial spoilage. Apples with a good wax coating keep longer. There was an old method of storing apples which used an oiled paper wrapping to reduce spoilage.
Whereas cellulose is a structural polysaccharide, starch is a storage polysaccharide. Thus starch is the major carbohydrate reserve in potatoes, whilst leafy plants, such as lettuce, contain relatively little starch and more cellulose. Starch differs from cellulose by containing α-1,4′ linked D-glucose units. These form a polymer known as α-amylose. The other major component of starch is amylopectin which contains the α-l,4′ linked D-glucose backbone together with some chain branching. Typically starch contains about 20–25% amylose and 75–80% amylopectin. Whereas cellulose has a fibrous structure, the α-amylose of starch with an α-1,4′ (axial-equatorial) linkage is wound into a more compact structure. The rigidity of this hydrogen-bonded structure in starch granules is destroyed as water is allowed to penetrate during cooking, for example, of potatoes so softening the vegetable.
Different enzyme systems are involved in the cleavage of cellulose and starch leading to differences in the ease with which they are digested. The human intestine can degrade starch but cellulose is only degraded by the bacteria in the gut and thus much of the cellulose acts as a dietary fibre.
Plants also produce a number of polysaccharides derived from other sugars, such as fructose, galactose, arabinose and xylose. These are known collectively as the ‘hemicelluloses’. One particular example is the fructan, inulin, which is produced by chicory and Jerusalem...