V. ZAGORIY
Chemical biology applies chemical methods, among them mass spectrometry, to solve biological problems. Although a description of the history of chemical methods in biology, finally leading to the establishment of mass spectrometry as a routine biochemical technique, cannot be given in a single chapter, we present selected discoveries, which, by demanding exact molecular mass measurements, have paved the way for biological mass spectrometry. We describe the discovery of vitamins D and E as an example of early chemical biology work. We highlight the development and application of chromatography, which became an essential auxiliary to mass spectrometry, and the role of this technology in the discovery of prostaglandins and pheromones. We briefly discuss the evolution of MSs, which enabled the analysis of organic molecules, and also show how interest in the mass spectrometry analysis of proteins led to the development of electrospray ionization (ESI), which has become the most popular ion source and has permitted the hyphenation of MSs to chromatography. We use the mechanism of developmental regulation in the roundworm Caenorhabditis elegans with small molecules as an example of an elegant study in the field of chemical biology, in which mass spectrometry has played a crucial role.
Chemical biology is a young discipline without a rigid definition. Nature Chemical Biology, a leading journal in the field, defines ‘chemical biology’ as the application of chemical methods to solve biological problems. Long before the term ‘chemical biology’ was coined, many fascinating discoveries in biochemistry had been made by applying chemical methods to biological phenomena. Biochemistry as a science can be traced back to the synthesis of urea by Wöhler in 1828 who, for the first time, was able to prepare a compound originating from living organisms through chemical synthesis.
Modern biochemistry relies heavily on organic mass spectrometry (MS), a method that originates from physics and that further evolved in the hands of chemists. Neither field describes the whole history of chemical methods in biology that finally led to the establishment of MS as a routine biochemical technique. The description of biological breakthroughs that were achieved with MS cannot be given in a single book, even less so a single chapter. In this introduction we present some selected discoveries that, by demanding exact molecular mass measurements, paved the way for biological MS, starting with the history of the discovery of lipophilic vitamins D and E at the beginning of the 20th century.
In 1925 it was known that two possibilities existed for the prevention of rachitis. One was the administration of cod liver oil and the other was irradiation of the skin with ultraviolet light. At that point it was considered that only two distinct antirachitic factors existed, until it unexpectedly turned out that UV-irradiation of food was also sufficient to prevent rachitis. It was concluded that a certain chemical factor exists, a pro-vitamin, which upon UV-irradiation is converted to an active antirachitic compound. In initial attempts to isolate this vitamin it was shown that the fraction that contained this pro-vitamin mainly contained cholesterol, suggesting that the pro-vitamin might also be a steroid. The biggest expert in the field of steroid chemistry at the time was Adolf Windaus from the University of Göttingen, who was invited to join the research in order to isolate and identify the antirachitic vitamin. Initially, the direct isolation of the vitamin from natural sources did not prove to be successful, so Windaus turned to an empirical approach, testing the antirachitic effect of known steroids after UV-irradiation. UV-treated cholesterol did not have any antirachitic effects (thus the expected vitamin D1 was not discovered), but ergosterol and 7-dehydrocholesterol produced antirachitic compounds after UV-treatment, which were named vitamins D2 and D3 respectively.1 Final proof of the vitamin D identity was obtained when Hans Brockmann, a student of Windaus, managed to isolate a natural antirachitic compound from tuna liver oil using liquid–liquid partitioning and column chromatography. Isolation was guided by an activity assay using rachitic rats and the isolated active compound was shown to be identical to the product of UV-treatment of 7-dehydrocholesterol.2
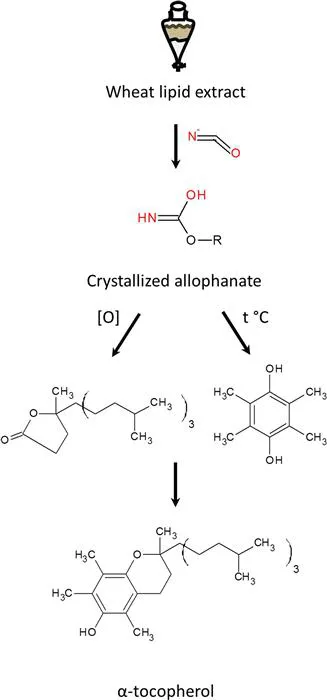
The history of the identification of vitamin E is closely related to vitamin D research. The existence of vitamin E was shown in an experiment by the laboratory of Herbert Evans at UC-Berkeley in 1922. They observed that rats that were fed a diet of purified protein, fat, carbohydrates, and vitamins A, B and C, which were already known at the time, were sterile,3 unlike animals given normal complete food. Isolation of the unknown factor necessary for reproduction was attempted by a number of research groups, mainly by means of liquid separation and adsorption chromatography. This approach did not prove to be successful. In 1932 Herbert Evans and two of his group members, Oliver and Gladys Emerson, travelled to Göttingen for a research stay at the laboratory of Alfred Windaus.4 After this visit, Evans and the Emerson couple made a new attempt at isolating vitamin E using an approach used by Adolf Butenandt, a student of Windaus, in many of his works on the isolation of natural compounds. Instead of isolating the intact vitamin E, they tried to crystalize it out of an enriched extract after chemical derivatization. Wheat germ was used as a starting material and an extract of non-saponified lipids turned out to be particularly active in supporting the reproductive function of rats. Evans and his colleagues did manage to crystalize the active compound out of this extract after treating it with cyanic acid,5 suggesting the presence of hydroxyl groups in vitamin E, which form a crystallizable allophonate. Final structure elucidation was performed by another Windaus student, Erhard Fernholz, who in 1938 showed that purified vitamin E under pyrolytic conditions decomposes into durohydroquinone and a hydrocarbon residue C19H38 (Figure 1.1). Under oxidative conditions, vitamin E produced a five-membered lactone bearing a hydrocarbon residue. Based on these findings Fernholz correctly identified the structure of vitamin E.6
Figure 1.1 Isolation and identification of α-tocopherol.
In these examples of vitamin research, low-efficiency isolation separation methods such as liquid–liquid partitioning or adsorption chromatography were used. These methods were appropriate for isolation of vitamins that were relatively abundant in the starting material, but as biological research started to deal with minute amounts of target compounds a need for new separation methods that could deal with much smaller amounts became clear.
At the same time as the group of Herbert Evans was developing an approach to crystallizing vitamin E, Archer Martin, a young PhD student from Cambridge, attempted isolation of non-modified vitamin E using counter-current liquid–liquid extraction. However, the group of Evans was the first to publish a report on its isolation and the results of Martin never appeared on paper.7 However, he built an extremely sophisticated counter-flow machine for his project, which gained him popularity as a solvent–solvent partitioning expert. As such, in 1938 he was approached by Richard Synge who was then working on the separation of amino acids. Among other methods, Synge tried the separation of amino acids using adsorption chromatography, which implements partitioning between a liquid mobile phase and a solid sorbent. However, the interaction of all amino acids with sorbents available at that time turned out to be very similar and did not permit separation of an amino acid mixture. Therefore, Synge sought the help of Archer Martin in trying to achieve amino acid separation using counter-current extraction. He had already shown that different acetylated amino acids had different partition coefficients between chloroform and water, and was looking for a way to use this fact for separation purposes. Despite certain success, such an approach turned out to be extremely cumbersome and technically demanding. A breakthrough was achieved when Martin decided to immobilize one liquid phase while moving the other. This was done by soaking silica gel with water and packing it into a column. Chloroform running through this column served as a mobile liquid phase. Partitioning of the analyte between the column and the mobile phase in this apparatus turned out to be very similar to the partitioning of analyte between two liquid phases.8 This method, which was later named partition chromatography, significantly improved the separation of acetylated amino acids, but it was still not good enough due to analyte adsorption on the silica, which interfered with the chromatography. Martin and Synge next tried using paper as a carrier for the immobilized water. Instead of packing paper soaked with water into a column they put a drop of the mixture to be separated onto the corner of a paper sheet and then “developed” it by putting one edge of the paper into a beaker with mobile phase. When the mobile phase reached the opposite edge, driven by capillary forces, the paper was taken out, dried and inserted with a neighboring edge into a different mobile phase, thus permitting “two-dimensional” separation. This method turned out to be so applicable to freeing amino acids that Synge used it to determine the sequence of gramicidin S. His research group first determined that this biologically active compound consists of valine, ornithine, leucine, phenylalanine and proline in equimolar amounts.9 The molecular mass suggested that gramicidin S is a decapeptide and its partial hydrolysis did yield a number of di- and tripeptides, which Synge identified using 2D partition chromatography on paper comparing the retention of hydrolysis products with synthetic dipeptides.10 Matching the sequence of dipeptides in the hydrolysate indicated a repeating pentapeptide sequence Val–Orn–Leu–Phe–Pro. The total sequence was suggested as being cyclic (Val–Orn–Leu–Phe–Pro)2 with the first valine linked to the last proline.11 This was the first-ever identified peptide sequence! Partition chromatography on paper was further used by Fred Sanger in his work on the sequence identification of insulin and generally became an extensively used analytical method. In the form of high-throughput thin layer chromatography, it is still used by biochemists today. In 1949 Archer Martin started working on the separation of fatty acids and came to the conclusion that a polar stationary phase did not permit a good enough separation with any of the available solvents. In order to improve the situation he tried to switch the chromatographic phases. Treatment of silicon dioxide with highly hydrophobic dichlorodimethylsilane covalently modified the former, changing it to an immobilized hydrophobic phase. Using water solutions of different alcohols as a mobile polar phase, Martin was able to achieve separation of long chain fatty acids.12 This separation method, later termed reversed-phase liquid chromatography (LC), is one of the most popular separation methods in bioanalytical chemistry today, and Martin and Synge received the 1952 Chemistry Nobel Prize for their work.
Reversed-phase chromatography proved to be an extremely powerful method for the separation of even small amounts of target substances from complex mixtures. One elegant example of the use of reversed-phase chromatography was the identification of the sex-attracting pheromone of the silkworm Bombyx mori, the first ever identified semiochemical. Male silkworm moths sense the presence of unfertilized female moths over very long distances. Up to the middle of 20th century this effect was explained as some kind of electromagnetic phenomena, ranging from infrared to X-ray radiation. Adolf Butenandt, at that time already a Nobel Prize winner for his work on sexual steroid hormones, made a suggestion that sexual attraction in silkworms is mediated by a chemical. Male silkworm moths when kept alone may not move for hours, but if a female moth is brought into the vicinity they start to move in a certain zigzag pattern. The same behavior is induced if female scent glands or their extracts are used instead. The group of Butenandt used extracts of scent glands obtained after the dissection of 500 kg silkworm female moths13 as a starting material for the isolation of the active compound. Every round of isolation was controlled using an activity assay based on the behavior of the male....