Science and technology are the cornerstones of the development of human society. Science provides humans with very powerful tools, the careless use of which endangers their world. The result could well be an environment inhospitable to human habitation. It is conceivable that without serious effort, this scenario will prevail and the further development of society could be jeopardized.
Chemistry is a very old discipline, with references to chemical transformations and debate about the nature of matter dating back to the times of the ancient Egyptians and Greeks. Modern chemistry began to emerge from alchemy in the seventeenth and eighteenth centuries, thanks to scholars such as Robert Boyle and Antoine Lavoisier, and made rapid advances in the following two centuries.
At the present time, the central role of chemistry in science is synthesis. The structure and bonding of molecules is at the core of the discipline, especially in organic chemistry, and using weaker intermolecular forces to assemble supra-molecules is a field with much still to explore. Understanding phenomena at the molecular level is vital to future innovation and invention.
The chemical sciences continue to be at the heart of multidisciplinary initiatives. Today, chemical tools — and the analytical tools chemists use — are especially pertinent to research in biology. For example, much of gene technology is chemistry. The chemical sciences provide the wide expertise for most scientific and technological developments and thus continue to make enormous contributions to social, cultural, economic and intellectual advances. Chemistry contributes to our well being, long life expectancy and economic prosperity. It satisfies the desire for better materials for everyday life and accommodation, for drugs to cure illnesses and improve health, for pure water, and a host of other human activities.
For this reason, energy and sustainable chemistry are key themes in current discussions about the future. Chemicals are present in all spheres of human life. Among other things, they are used as pharmaceuticals, pesticides, detergents, fertilizers, dyes, paints, preservatives and food additives. The first and most influential description of the dangers related to chemicals in the environment is found in Rachel Carson’s Silent Spring, published in 1962.1
Synthetic chemicals end up in the environment in many different ways. The chemical industry is a point source of emissions which create changes around that point. In everyday life the constituents and ingredients of consumer or household products and other open applications emit chemicals into the environment via non-point sources. Chemicals and their compositions do degrade and break down into water, carbon dioxide and inorganic salts, but very often the degradation is incomplete. Unknown transformation products can result from such biological and chemical processes as hydrolysis, redox reactions or photolysis. These unknown chemical entities remain in the environment and can be toxic to humans and environmental organisms. The latter situation is more serious as there is usually much less knowledge about the longevity and effects on the environment of the final transformation products than there is about the parent compounds. Even if there is some degree of degradation, the parent compounds will nevertheless remain at constant levels in the environment if the input rate is higher than their rate of degradation or mineralization. This situation has to do with the persistency of chemicals. Persistency is one of the most important criteria in the environmental assessment of chemicals.
Polychlorinated biphenyls (PCBs) are a classic example of persistent pollutants. PCBs were synthesized for the first time in 1877, and as early as 1899 severe health problems (chloracne) associated with the handling of PCBs were reported. Since then, the poisoning of rice oil by these compounds and their neurotoxic effects and carcinogenicity have been described in detail. Despite this knowledge, it was not until 1999 that PCBs were completely banned within the EU – 100 years after the first reports of their severe toxicity. This example clearly demonstrates that it is not only the time lag of the impact of the chemicals on environmental processes, but also the time lag of economic and political systems which has a significant effect.
In 1995, the Governing Council of the United Nations Environment Programme (UNEP) called for global action to assess the effects of POPs (persistent organic pollutants). The twelve worst offenders are known as the “dirty dozen” and include eight organo-chlorine pesticides: aldrin, chlordane, DDT, dieldrin, endrin, heptachlor, mirex and toxaphene; two industrial chemicals: hexachlorobenzene (HCB) and the polychlorinated biphenyl (PCB) group; and two groups of industrial byproducts: dioxins and furans. It became clear that these POPs were deadly and that urgent global action was needed. The result was the Stockholm Convention on Persistent Organic Pollutants in May 2001.2 The Convention outlaws the dirty dozen and also establishes a system to track additional substances that can be classified as POPs, to prevent the development of new problem chemicals.
According to the Stockholm convention, a half-life of more than 50 days in water is set as a criterion for POPs. Recent research has demonstrated that chemicals which are less persistent and have a higher polarity than PCBs are distributed globally as well, and can also accumulate in humans.
An example of pollutants with a much shorter history are the inert organohalogen compounds, known as freons. They were initially developed in the early 20th century as an alternative to the toxic gases that were used as refrigerants, such as ammonia, chloromethane and sulfur dioxide. These compounds, which contain only chlorine, fluorine, and carbon, are called chlorofluorocarbons (CFCs). Each freon product is designated by a number. For instance, Freon-11 is trichlorofluoromethane and Freon-12 is dichlorodifluoromethane. The most common is Freon-113, trichlorotrifluoroethane, used as a cleaning agent.
In the 1970s, scientific evidence showed that human-produced chemicals are responsible for observed depletions of the ozone layer. In 1978, the United States, Canada and Norway enacted bans on aerosol sprays containing CFC that are thought to damage the ozone layer. After the discovery of the Antarctic ozone hole in 1985, CFC production was sharply limited as of 1987 and was phased out completely by 1996 according to an international treaty. The Montreal Protocol on Substances that Deplete the Ozone Layer classifies Freon-11 and Freon-12 as Annex A substances, and bans their production and consumption.3 It has now been confirmed that the upper atmosphere ozone depletion rate has slowed significantly during the past decade.
The interim replacements for CFCs are hydrochlorofluorocarbons (HCFCs), which contain chlorine that depletes stratospheric ozone, but to a much lesser extent than CFCs. Ultimately, hydrofluorocarbons (HFCs) will replace HCFCs with essentially no ozone destruction, although all three groups of halocarbons are powerful greenhouse gases.
The Montreal Protocol on Substances That Deplete the Ozone Layer is a landmark international agreement designed to protect the stratospheric ozone layer. The treaty was originally signed in 1987 and substantially amended in 1990 and 1992. The Montreal Protocol stipulates that the production and consumption of compounds that deplete ozone in the stratosphere – chlorofluorocarbons (CFCs), halons, carbon tetrachloride and methyl chloroform – are to be phased out by 2000 (2005 for methyl chloroform).
Chemistry, and the application of chemical technology, can be practised and useful materials synthesised in many ways, some of which are safer for the environment and human health than others. Safety for humans and the environment, as well as the economical use of resources, must be of utmost consideration in the development of new chemical processes. In other words, the true cost of resources and waste must be included in every calculation and comparison. The mind-set in chemistry must be changed from waste treatment to the prevention of waste generation.
New environmental regulations, and a growing social consciousness with regard to the protection of nature, have nudged the chemical sciences and industry toward a new framework in which pollution prevention is the central consideration. This fact presents an enormous technological and scientific challenge that requires the development of innovative products and tools to minimize the impact on the environment. Nevertheless, the same standards of quality and efficiency need to be retained in order to continue scientific advancement within a sustainable framework.
Concerned individuals have raised these questions and citizens are becoming aware of the critical situation. This is also true of chemical scientists and engineers and the chemical industry where a new wind is blowing. A comprehensive and accurate understanding of what constitutes the sustainable development of human civilization is required, taking into account the ecological, economic and social dimensions. This is simple in principle but much more difficult in practice.
In the long term, chemicals end up in the environment. Therefore, an important requirement is to focus not only on the optimization of the chemical synthesis of a molecule and its use, but also to take into account the molecules themselves and their effect on the environment. To quote Anastas and Warner in Green Chemistry: Theory and Practice: “Chemical products should be designed so that at the end of their function they do not persist in the environment and do break down into innocuous products”.4 Designing chemicals to meet both the requirements of their application and environmental considerations, throughout their life cycle, is ambitious and quite new. It must, however, become an essential part of the core of chemistry and pharmacy.
Agenda 21, Chapter 35 of Science for Sustainable Development,5 focuses on the role and the use of the sciences in supporting the prudent management of the environment and development for the daily survival and future development of humanity. “The sciences should continue to play an increasing role in providing for an improvement in the efficiency of resource utilization and in finding new development practices, resources and alternatives. There is a need for the sciences to constantly reassess and promote less intensive trends in resource utilization, including less intensive utilization of energy in industry, agriculture and transportation. Thus, the sciences are increasingly being understood as an essential component in the search for feasible pathways towards sustainable development”.
Scientists must develop their basic and applied research topics in accordance with the sustainable development of society. This also applies to chemistry and chemical research topics. Indeed, chemistry – expressed as the courage and curiosity to discover and formulate new materials and compounds to improve human life – plays the most important role in this process. However, chemistry is changing very slowly. Obviously, chemical companies are accustomed to petrochemicals and are reluctant to use alternative renewable feedstocks which may not be well suited to the usual petrochemical processing.
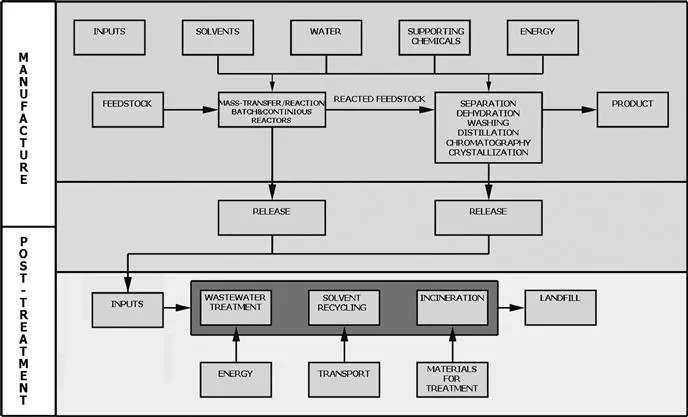
A general process scheme is presented in Figure 1.1 which illustrates the complexity and importance of factors other than the product outcome.
Figure 1.1 General process scheme.
The following describes a pharmaceutical industry manufacturing process based on this scheme. The normal manufacturing yield from a single stage ranges between 35 and 95%, with an average yield of 86% The typical primary manufacturing process involves six stages with an overall yield of 30–40%. Overall yield does not capture the use of reagents, solvents and catalysts. If these are included, the average total materials use is 16 kg/kg of stage product (intermediate)....