![]()
CHAPTER 1
Introduction
1.1 CLUSTERS AND NANOPARTICLES
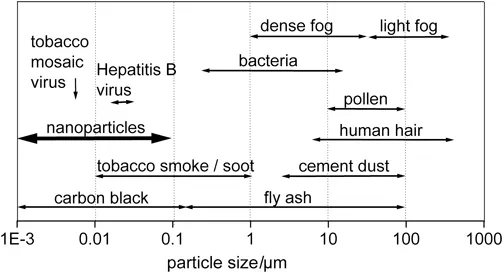
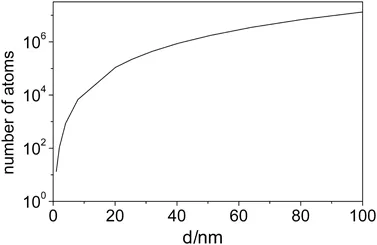
Particles with a size in the range between 1 and 100 nm are normally regarded as nanomaterials. Figure 1.1 shows the size of nanoparticles in comparison with other small particles. In general, nanomaterials may have globular, plate-like, rod-like or more complex geometries. Near-spherical particles which have a countable number of atoms are typically called clusters. The number of atoms in a cluster or nanoparticle increases greatly with its diameter. This is demonstrated in Figure 1.2 for the example of sodium clusters. At 1 nm diameter there are 13 atoms in a cluster and at 100 nm diameter the cluster can accommodate more than 107 atoms. Clusters may have a symmetrical structure which is, however, often different in symmetry from that of the bulk. They may also have an irregular or amorphous shape. As the number of atoms in a cluster increases, there is a critical size above which a particular bond geometry that is characteristic of the extended (bulk) solid is energetically preferred so that the structure switches to that of the bulk.
Figure 1.1 Typical size of small particles. The regime below 0.1 µm corresponds to the dimension of nanoparticles.
Figure 1.2 Number of sodium atoms in a spherical cluster of diameter d.
It is below a dimension of 100 nm where properties such as melting point, colour (i.e. band gap and wavelength of optical transitions), ionisation potential, hardness, catalytic activity and selectivity, or magnetic properties such as coercivity, permeability and saturation magnetisation, which we are used to think of as constant, vary with size. We basically distinguish two types of variation as a function of size:
- Scalable effects: Surface atoms are different from bulk atoms. As the particle size increases the surface-to-volume ratio decreases proportionally to the inverse particle size. Thus, all properties which depend on the surface-to-volume ratio change continuously and extrapolate slowly to bulk values. For clusters and nanoparticles consisting of spherical units (atoms, ions or small molecules) the surface-to-volume ratio relates to the average coordination number. For soft matter structures which often consist of long surfactant molecules the surface curvature is a better measure.
- Quantum effects: When the molecular electronic wave function is delocalised over the entire particle then a small, molecule-like cluster has discrete energy levels so that it may be regarded like an atom (sometimes called a superatom). The simplest model for it is that of a particle in a box. Adding more atoms to the cluster changes the size of the box continuously so that the energy levels close up to some extent. More importantly, adding more atoms means adding more valence electrons to the system. Thus, whenever a shell of sometimes multiply degenerate energy levels is filled the next electron has to be accommodated in the next shell of higher energy. The situation is analogous to the evolution of properties with increasing atomic number in the periodic table. Filled shells represent a particularly stable configuration. Properties such as ionisation potential and electron affinity are well known to display a discontinuous behaviour as one moves along the periodic table. For clusters consisting of atoms with strongly overlapping atomic orbitals, i.e. for metals and semiconductors, the situation is analogous.
In metals, collective motions of conduction electrons, called plasmons, may be excited. In an analogous way, internal vibrational wave functions couple to coherent motions, called vibrons. Both plasmons and vibrons are size-dependent.
Quantum effects are more pronounced for small clusters and often superimposed on a smoothly varying background of a scalable effect. Clusters are interesting intermediates between single atoms and bulk matter and represent a natural laboratory to ‘see both ends from the middle’.
1.2 FEYNMAN’S VISION
On 29 December 1959, at the annual meeting of the American Physical Society, Richard Feynman addressed the audience with his visionary and by now historical and legendary lecture under the title There is Plenty of Room at the Bottom: Invitation to Enter a New Field of Physics.1 With this talk on the problem of manipulating things on a small scale, Feynman opened the field of nanotechnology. Today, more than five decades later, the field is finally seen to really take off. It is amazing how closely some of the key developments follow Feynman’s vision. ‘Why cannot we write the entire 24 volumes of the Encyclopaedia Britannica on the head of a pin?’ he asked. We know how successful information technology has been in its work toward this goal and we should be aware of how much this has influenced our lives. ‘Make the electron microscope a hundred times better,’ Feynman said. The development of the atomic force microscope was one of the milestones on the way not only to observe but also to manipulate in atomic dimensions. Amazingly, Rohrer and Binnig achieved this goal with their cantilever-based instrument in a single step, rather than by a hierarchy of smaller and smaller robots − training an ant to train a mite − as Feynman suggested. In 1986 the two scientists were honoured for their achievement with the Nobel Prize in Physics. It is quite obvious today that the invention of the scanning tunnelling microscope finally triggered the boom in nanotechnology, in which the direct observation of very small-scale structures down to individual atoms is essential. Obviously, seeing things directly is more convincing for vision-based beings than just having measurements which are in agreement with a model.
Some of the inspiration came from biology. Feynman understood that information is stored on a molecular level in biology, that cells manufacture substances and operate on a small scale, that the human brain is a wonderful and efficient miniaturised computer. ‘Consider the possibility that we too can make a thing very small which does what we want, an object that manoeuvres at that level,’ he suggested. While his talk was primarily technology oriented, he knew that physics, chemistry, biology and engineering are all relevant and must all be involved. He realised that making things smaller was not just a technological problem of scaling down. He saw that certain things changed principally. Magnetism, for example, is a cooperative phenomenon and involves domains which cannot be reduced down to an atomic size. Atoms differ from the bulk in their quantum nature. Most significantly, Feynman predicted that when we have some control of the arrangement of things on a small scale we will get an enormously greater range of possible properties that substances can have. It is exactly the arrangement of things on a small scale which is the foundation for all the excitement about nanomaterials and for the success of modern materials science.
1.3 MICROSCOPY AT ATOMIC RESOLUTION
It is amazing how much chemists have learnt about the structures and transformations of molecules without having ever directly seen them. The entire knowledge was developed via model-based interpretations of a multitude of experiments. During the last century, crystallography, spectroscopy and quantum chemical calculations were added to the earlier methods which consisted to a large extent in synthesising derivatives of a compound for which the structure had to be determined, and they made the life of chemists far easier. Remarkably, despite the fact that with the exception of quantum chemistry these methods provide information for an ensemble average, they have normally led to consistent images of molecular structures, and in the majority of cases nobody doubts that these structures are correct.
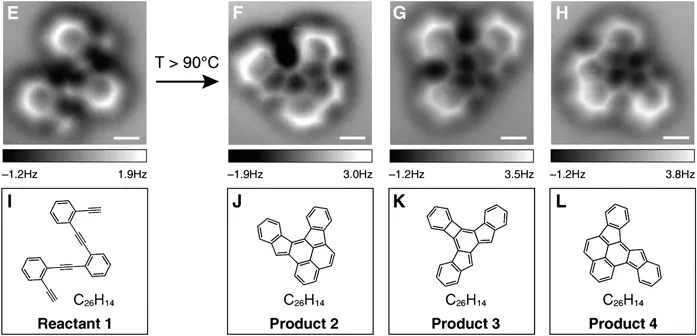
As a student, the author of this book was taught that for fundamental reasons it will never be possible to resolve individual atoms in microscopic experiments. Since the invention of scanning tunnelling and atomic force microscopy, however, this is no longer true. In fact it has recently become possible to place molecules on a flat surface and let them react while they are monitored by atomic force microscopy (AFM).2 It turns out that they look exactly the way we have learnt to draw them on a sheet of paper. Figure 1.3 demonstrates how non-contact atomic force microscopy can image even the internal bond configurations of oligo-(phenylene-1,2-ethynylenes) on an Ag(100) surface and their changes as they undergo a series of quite complex thermally induced cyclisation reactions.
Figure 1.3 Atomic force microscopy (AFM) images of oligo-(phenylene-1,2-ethynylene) (E) and three of its reaction products (F, G, H) after thermal annealing on an Ag(100) surface. All images were acquired at constant height in non-contact mode that monitors the short-range Pauli repulsion between the observed molecule and the oxygen of a single carbon monoxide molecule that was attached to the apex of the AFM tip. Schematic representations of the molecular structures are given below the AFM images (I–L).
(Reproduced with permission from Ref. 2.)
REFERENCES
1. R. P. Feynman, Eng. Sci., 1960, 23, 22; J. Micromech. Syst., 1992, 1, 60. www.zyvex.com/nanotech/feynman.html
2. D. G. de Oteyza, P. Gorman, Y.-C. Chen, S. Wickenburg, A. Riss, D. J. Mowbray, G. Etkin, Z. Pedramrazi, H.-Z. Tsai, A. Rubio, M. F. Crommie and F. R. Fischer, Science, 2013, 340, 1434.
![]()
CHAPTER 2
Bulk and Interface
2.1 GRADIENTS NEAR SURFACES
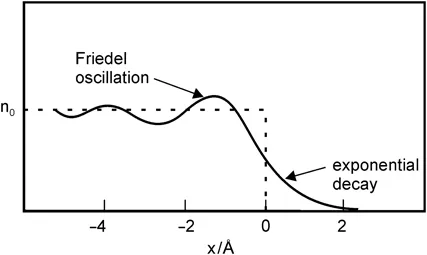
A simplistic view distinguishes between gaseous, liquid and solid phases of a single chemical component, and it assumes that there is a sharp phase boundary where properties change discontinuously between two homogeneous neighbouring phases. In nature there are of course no discontinuities, and when we focus our view onto the interface we first find that it is corrugated on a level of atomic or molecular dimensions. More importantly, although the chemical potential of an equilibrated system is by definition the same in the bulk of both phases and does not change through the phase boundary, most other properties depend on the phase, but they change continuously along the coordinate perpendicular to the interface, often with superimposed oscillations. An example is given in Figure 2.1.
Figure 2.1 Schematic drawing of the charge density distribution (Friedel oscillations) across the surface of a spin polarised electron gas representing the conduction electrons of a metal. (Adapted from Ref. 1, see also Refs. 2–4, used by permission of John Wiley & Sons, Inc.)
Let us assume that we cleave a perfect single crystal in vacuum. After cleavage, the density drops to zero instantaneously at the new interface, and the atoms or molecules near the surface experience an asymmetric interaction because some of the partners on one side have disappeared. Thus, they have to adjust to find a new balance of interacting forces. This is achieved by relaxation of the lattice. As a consequence, the local lattice parameters change, and the density becomes a function of distance from the surface. It is plausible that this gradual change in density is paralleled by changes in most other properties. The wave function is modified, bond...