1.1 Drug Impurities, Degradants and the Importance of Understanding Drug Degradation Chemistry
A drug impurity is anything that is not the drug substance (or active pharmaceutical ingredient, API) or an excipient according to the definition by the US Food and Drug Administration (FDA).1 Impurities can be categorized into process impurities, drug degradation products (degradants or degradates), and excipient and packaging-related impurities. Process impurities are produced during the manufacture of the drug substance and drug product, while degradants are formed by chemical degradation during the storage of the drug substances or drug products. The storage conditions are typically represented by the International Conference on Harmonisation (ICH)- and World Health Organization (WHO)-recommended stability conditions which simulate different climatic zones of the world.2,3 Certain process impurities can also be degradants, if they continue to form in storage under stability conditions. Packaging-related impurities, also called leachables, are typically various plasticizers, antioxidants, UV curators, and residual monomers that leach out of the plastic or rubber components and labels of the package/container of a drug product over time.
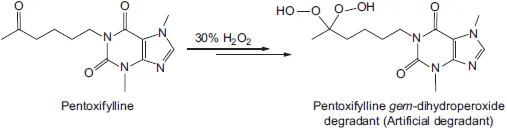
Those process impurities that are not degradants may be controlled or eliminated by modifying or changing the process chemistry. On the other hand, control or minimization of drug degradants requires a clear understanding of the drug degradation chemistry, which is not only critically important for developing a drug candidate but also for maintaining the quality, safety, and efficacy of an approved drug product. Specifically, knowledge of drug degradation is not only vital for developing adequate dosage forms that display favorable stability behavior over the registered product shelf-life, but is also critical in assessing which impurities would be most likely to be significant or meaningful degradants so that they can be included in the specificity mixture when developing and validating stability-indicating analytical methodologies. A common problem in the development of stability-indicating HPLC methods using stress studies (or forced degradation) is a lack of proper evaluation if the stress-generated degradants would be real degradants or not. From a practical point of view, the real degradants are those that can form under long term storage conditions such as the International Conference on Harmonisation (ICH) stability conditions.2 On the other hand, various artificial degradants can be generated during stress studies, in particular when excessive degradation is rendered or the stress conditions are not consistent with the degradation pathways of the drug molecule under the usual stability conditions. For example, forced degradation of a ketone-containing drug, pentoxifylline, using 30% hydrogen peroxide at room temperature for eight days produced a geminal dihydroperoxide degradation product (Scheme 1.1).4 This compound is highly unlikely to be a real degradant of the drug product.
Scheme 1.1
This book is devoted to increasing our understanding and knowledge of the organic chemistry of drug degradation. The knowledge derived from this endeavor should also be beneficial for the elucidation of drug metabolite structures and bioactivation mechanisms. Most drugs undergo at least certain level of metabolism,5 that is, chemical transformation catalyzed by various enzymes. Except in the case of pro-drugs, drug metabolites can be considered as drug degradants formed in vivo. Chemical degradation and drug metabolism can produce the same degradants, even though they may go through different reaction intermediates or mechanisms. In vitro chemical reactions have been used to mimic enzyme-catalyzed drug metabolism processes, in order to help elucidate the enzymatic mechanisms for the catalysis.6 On the other hand, understanding the mechanisms of drug metabolism may also facilitate the elucidation of drug degradation pathways in vitro.
Regardless of their origins, certain drug degradants can be toxic, which is one of the main contributors to undesirable side effects or adverse drug reactions (ADR) of drugs.7 In the early stage of drug development, the degradants (including metabolites) and degradation pathways (or bioactivation pathways in the case of reactive metabolites) of a drug candidate need to be elucidated, followed by toxicological evaluation of these degradants. Dependent upon the outcome of the evaluation, the structure of the drug candidate may have to be modified to avoid the formation of a particular toxicophore based on the understanding of the degradation chemistry (or bioactivation pathways) elucidated. Failure to uncover toxic degradants, usually the low level ones, in the early development stage can lead to hugely costly failure in later stage clinical studies or even withdrawal of an approved drug product from the market.
1.2 Characteristics of Drug Degradation Chemistry and the Scope of this Book
The vast majority of therapeutic drugs are either organic compounds or biological entities. The latter drugs include protein and nucleic acid (RNA and DNA)-based drugs which are biopolymers comprising small molecule building blocks. This book focuses on the organic chemistry aspect of drug degradation, in particular, the mechanisms and pathways of the chemical degradation of both small and large molecule drugs under real life degradation scenarios, as represented by the usual long term stability conditions. Stress studies or forced degradation can help elucidate the structures of real degradants and the degradation pathways of drugs. Nevertheless, caution needs to be taken in differentiating the real and artificial degradants. This subject will be discussed in detail in Chapter 8, Strategies for Elucidation of Degradant Structures and Degradation Pathways.
Drug degradation chemistry differs from typical organic chemistry in several ways. First, the yield of a drug degradation reaction is usually very low, from approximately 0.05% to a few percentage points at the most. Dependent upon the potencies and maximum daily dosages of the drugs, ICH guidelines require that the impurities and/or degradants of a drug be structurally elucidated, once they exceed certain thresholds, which are typically between 0.05% and 0.5%, relative to the drug substances.8,9 For potential genotoxic impurities, they need to be characterized and controlled at a daily maximum amount of 1.5μg for drugs intended for long term usage.10 Such low yields would be meaningless from the perspective of the regular organic chemistry. Second, due to the low yields and limited availability of samples, particularly stability samples of formulated drugs, the quantity of a drug degradant is usually extremely low, posing a serious challenge for its isolation and/or characterization. Despite the advent of sensitive and powerful analytical methodologies such as high resolution tandem liquid chromatography-mass spectrometry (LC-MS/MS), liquid chromatography-nuclear magnetic resonance (LC-NMR), and cryogenic micro NMR probes, the identification of drug degradants remains one of the most challenging activities in pharmaceutical development.11 Third, the typical conditions and “reagents” of drug degradation reactions are limited in scope. For example, the ICH long term stability conditions for different climatic zones specify the requirements for heat and moisture (relative humidity, RH), for example, 25°C/60% RH and 30°C/65% RH, while the ICH accelerated stability condition requires heating at 40°C under 75% RH. In addition to moisture, the other most important “reagent” in drug degradation reactions is molecular oxygen. Since molecular oxygen is ubiquitous and difficult to remove from drug products, oxidative degradation of drugs is one of the most common degradation pathways. Often, the impact of molecular oxygen can be indirect. For example, a number of polymeric drug excipients such as polyethylene glycol (PEG), polysorbate, and povidone, are readily susceptible to autooxidation, resulting in the formation of various peroxides including hydrogen peroxide.12–14 These peroxides can cause significant drug degradation once formulated with drug substances containing oxidizable moieties. In contrast, reductive degradation is rarely seen in drug degradation reactions owing to the lack of a reducing agent in common drug excipients that is strong enough to cause meaningful reductive degradation. Other possible “reagents” in drug degradation reactions are usually limited to drug excipients and their impurities. For example, excipients consisting of oligosaccharides and polysaccharides with reducing ends, such as lactose and starch, are frequently used in drug formulation. The aldehyde functionality of these excipients can react with the primary and secondary amine groups of drugs to undergo degradation via the Maillard reaction. This topic will be covered in Chapter 5, Drug–Excipient Interaction and Adduct Formation.
As indicated above, this book focuses on the organic chemistry of drug degradation, in particular, the mechanisms and pathways of the chemical degradation of both small and large molecule drugs under real life degradation scenarios. Owing to the variety of dosage forms of formulated drugs, degradation of drugs can occur in various states including solid (tablets, capsules, and powders), semi-solid (creams, o...