![]()
Section IV
Function and Effects
![]()
CHAPTER 7
Fluoride Accumulation in Crops and Vegetables: Indian Perspectives
SRIMANTA GUPTA*a AND DALI MONDALa
aDepartment of Environmental Science, The University of Burdwan, Burdwan, 713 104, West Bengal, India
*E-mail:
[email protected] 7.1 Introduction
The availability of clean water is essential. It serves as the basic component in different spheres of human life for a large number of habitants (Ayoob and Gupta, 2006). In many regions of the world populations are dependent on groundwater for agriculture and drinking (Amini et al., 2008; Gupta and Banerjee, 2009). Unfortunately, groundwater may be contaminated from natural sources by aquifer rocks. One of the most widespread contaminant is fluoride (F) and it is estimated that many millions of people are affected worldwide (WHO, 2008).
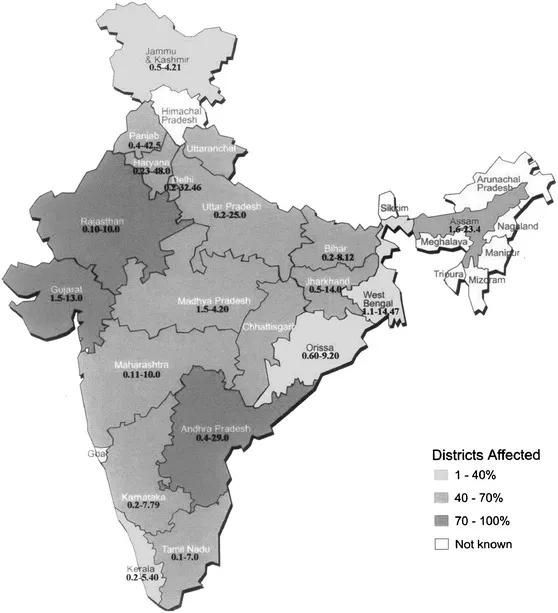
It has been estimated that nearly 25 million people in India are already affected by F while over 66 million more, including 7 million children, in 20 states (Figure 7.1) of the country are susceptible to it. Out of the total of 569 districts of India, 205 (36%) have F contamination in their drinking water. Fluoride contamination in the drinking water of these districts is mostly geogenic in nature though there are a few pockets where brick kiln, alumina, phosphate and fertilizers industries are the culprit. As per the records of the Central Ground Water Board (CGWB), Government of India, all districts of Rajasthan, 15 districts of Andhra Pradesh, 27 districts each of Orissa and Gujarat, 18 districts of Uttar Pradesh, 13 districts of Punjab, 12 districts each of Karnataka and Haryana and 10 districts each of Madhya Pradesh and Maharashtra have high F concentration (above the permissible limits). In a country like India, the majority of the population (85%) lives in villages and depends mainly on groundwater resources both for drinking and agriculture purpose. Approximately 65% (50–80%) of the irrigated land is under groundwater consumption (Raju, 1998).
Figure 7.1 Fluorosis affected states in India. The number within each state is the fluoride range in drinking water. Rajasthan, Gujarat and Andhra Pradesh are the most affected states in India. (Source: UNICEF State of Art Report, 1999 and FR and RDF data bank.)
Fluoride is not an essential element for plants but is essential for animals and humans. The continuous ingestion by animals in excessive amounts of F may lead to fluorosis and the suboptimal levels in the diet can have an equally damaging effect (Kabata-Pendias, 2001). The World Health Organisation (WHO, 1984) and Bureau of Indian Standards (BIS, 2003) have laid down the maximum permissible limits of F in drinking water as 1.5 mg L−1and 1.0 mg L−1, respectively, but there is no stringent threshold limits of F in soil and plants above which the ingestion may be detrimental to human health. The major part of F ingested in areas endemic to fluorosis is water, although some food materials contribute considerable amounts to total intake (Singh et al., 1993). Crops grown in close proximity to certain factories, especially those producing phosphate fertilizer, steel, aluminum, magnesium, glass and bricks contain more F since F and heavy-metal-containing dust emitted into the air settle on plants (Oelschlager, 1974). The gaseous F emitted from these industrial sources is known to have phytotoxic effects on plants. Plants can also incorporate F from contaminated soils (Arnesen, 1997). The fluoride thus absorbed is translocated to the shoots, causing physiological, biochemical and structural damage and even cell death, depending on the concentration in the cell sap (Miller, 1993). Some plants accumulate F even at higher concentration (up to 4000 µg F g−1) do not show signs of toxicity (Sheldrake et al., 1978). Most other plants show sign of toxicity at much lower concentration (Brewer, 1966), while some species are extremely sensitive to concentration <20 µg F g−1 (Istas and Alaerts, 1974). Fluoride is more soluble in acid soils due to which its uptake by plants is enhanced. Liming the soil with calcium oxide reduces F accumulation (Bear, 1954). Plants grown in clay loam take up less F than those grown in sand. Plants do not require F, and tissues concentration from uncontaminated soils rarely exceed 30 mg kg−1, dry mass (Kabata-Pendias, 2001). The fluoride content of both leafy and root vegetables usually do not differ appreciably from those of cereals, with the exception of spinach that is usually enriched in F and it is known as a good accumulator of F (Madhavan and Subraminian, 2006). Among items of plant origin, tea leaves contain significant amounts (30–300 mg g−1 dry weight) of F. Bioavailability of F to plants could be influenced by several metal ions due to complexation, precipitation, pH changes, etc.
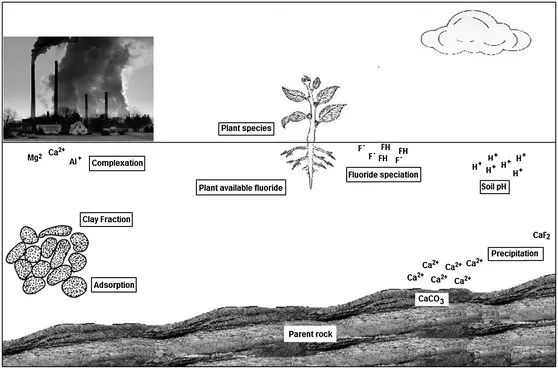
From an Indian perspective very limited field-based research work has been done in the aspect of F accumulation in crops and vegetables (Gupta and Banerjee, 2011; Saini et al., 2013). Most of the research studies have been done in the aspect of physiological and biochemical effects and that too also in the laboratory condition or in pot culture (Brahmbhatt and Patel, 2013). It is thought that substantial amounts of F may be ingested by crops and vegetables cultivated with F-contaminated irrigation water. In some states of India particularly Rajasthan, Uttar Pradesh and Andhra Pradesh F-contaminated groundwater is the major source of irrigation water for cultivation (Gupta and Banerjee, 2009). Fluoride uptake by plants depends on the biogeochemical factors, affecting its availability in soils. With this background this book chapter focuses on the fate of F in soils, its uptake, and distribution and accumulation in different crops and vegetables of Indian origin. Additionally, a separate case study is also discussed for better understanding of its accumulation scenario in different crops and vegetables as well as its quantitative intake thorough diet. Figure 7.2 illustrates the factors and processes that influence the F availability in soils, irrigated with F contaminated water discussed in this chapter.
Figure 7.2 Schematic diagram showing factors and processes influencing fluoride availability in plants. Fluoride emitted from industries become complexed with Al3+, Mg2+ and Ca2+. In acidic soil F became available to plant. Fluoride adsorbed at the surface of clay particle by adsorption process. Presence of excess Ca2+ may induce precipitation of F as CaF2 by precipitation process. (Source: own artwork.)
7.2 Fluoride in Soil
For all soils, it is the soluble F content that is biologically important to plants and animals. The solubility of F in soil is controlled mainly through F adsorption by inorganic constituents of the soil and soil pH (Loganathan et al., 2003). In normal soil, the F is strongly adsorbed to the soil (Barrow, 1986) and hence plant uptake of F is generally minimal (McLaughlin et al., 1997). The greater solubility of F under acidic conditions was explained by the formation of AlFx complexes, whereas under alkaline conditions by desorption of free F as a result of repulsion by the negatively charged surfaces (Wenzel and Blum, 1992).
7.2.1 Mobility of Fluoride in Soil Solution
The mobility of F in soils depends on various factors such as initial concentration of F, solubility of F-bearing minerals, types of soil, pH of soil and other dissolved cation/anions in the soil solution. The solubility of F is controlled by equilibrium with F-containing minerals. In calcareous soil (pH ranges 7.0 to 8.5) a continuous supply of soluble calcium will induce the fluorite precipitation when this type of soil is irrigated with high-F water. The presence of large amounts of clay size fraction and aluminum hydroxide also affect the absorption of F in the soil (Arnesen and Krogstad, 1998). Aluminium hydroxide has a pH range of 7.1 to 9.5 and alumina has PZC of 8.0 (Mullar, 2005). So F has a high affinity to adsorb on these minerals in the neutral pH range. However, anion exchange is the major mechanism for the retention of F on aluminum hydroxide. The general reaction of anion exchange of F with minerals (Huang and Jackson, 1965) can be written as:
Mineral − OH + F → Mineral − F + OH−
The presence of clay minerals in soil plays an important role in retaining F in soils in the pH range between 5.5 and 6.5.
7.2.2 Pathways of Fluoride Uptake by Plants
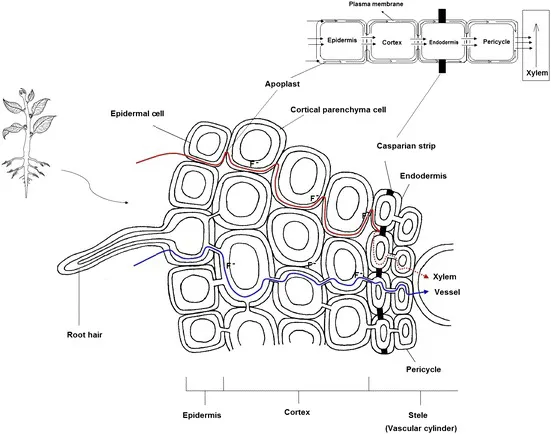
Most of the soluble F fraction in the soil is transported to plants through an apoplastic transport mechanism in the root systems (Figure 7.3). This mechanism is more dominant because cell walls also having negative charge promote the transport of negatively charged F ions. Apart from this, the low permeability of F through cell membrane also hinders diffusion in the cells. The apoplastic (nonliving) pathway provides a route toward the vascular stele through free spaces and cell walls of the epidermis and cortex. An additional apoplastic route that allows direct access to the xylem and phloem is along the margins of secondary roots. Secondary roots develop from the pericycle, a cell layer just inside the endodermis. The endodermis is characterized by the casparian strip, a suberized layer that hinders F movement in the symplast in order to enter the vascular system. Since secondary roots grow through the endodermis, a direct pathway to the xylem and phloem is available that bypasses the casparian strips and allows F to enter the vascular system without moving into the symplast (living tissue).
Figure 7.3 Pathways of fluoride in the root. This figure illustrates apoplastic pathways of fluoride through the root cell. (Source: own artwork.)
7.2.3 Factors Affecting Plant Uptake of Fluoride
In general, it is expected that F would be more readily taken up by plant at a pH le...