The Hebrew University of Jerusalem, Institute of Chemistry and the Lise Meitner-Minerva Center for Computational Quantum Chemistry, Edmond J. Safra Campus, Givat Ram, Jerusalem, 91904 Israel
1.1 Introduction
One of the fundamental territories of chemistry is the chemical bond, the glue from which an entire chemical universe is constructed, resulting in a rich and enticing variety of species held together by stabilizing interactions. Let me first define the interactions and the manner in which they will be classified in this introductory chapter.
There are interactions that pair-up or delocalize electrons over a two or more atoms, and thereby give rise to entities we call molecules. This chapter refers to these interactions as chemical bonds. The chemical bond defines a chemical identity. It also accounts for the ‘magic’ of chemistry, whereby one molecule disappears and a new one appears. Take for example the soft gray solid, sodium, (Na)s, and mix it with the yellow-greenish gas, chlorine (Cl2). And within seconds, lo and behold, you get a white solid, (Na+Cl−)s. All three species have distinct identities, being defined by their chemical bonds, and as such the magic is a manifestation of breaking bonds and making new ones. Clearly, then, the term chemical bond defines the molecule, and hence also the chemical identity. Usage of this term is also a tribute to Gilbert Newton Lewis who invented it 100 years ago1as a quantum unit of bonding in molecules. By so doing, Lewis has ushered “the electronic-structure revolution in chemistry”.2
There are however, stabilizing interactions also between and among molecules with saturated valences, such as hydrogen bonds, dipole–dipole and dispersion interactions, and so on. These interactions are responsible for another type of ‘magic’- the magic of aggregation, crystallization, gelation, coagulation, micelle formation, self-assembly, and evolution of large mesoscopic bodies. Consider for example discrete water molecules in the gas phase (vapor). As you lower the temperature, gradually you see water appearing as a liquid, and as temperature is further lowered, lo and behold, the liquid becomes a beautiful translucent solid, ice. Thus, the interactions between the discrete molecules bring about the formation of mesoscopic and macroscopic matter, and as such, we refer to these cohesive forces as intermolecular interactions.
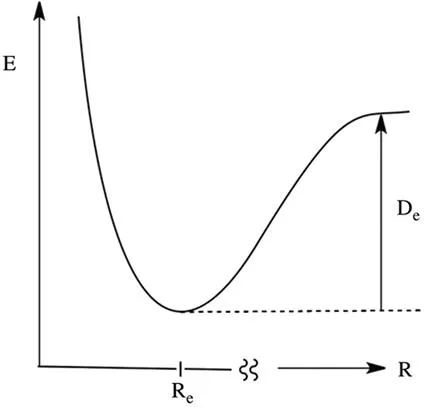
Despite the identity of the forces involved in these two kinds of interactions and the fact that their energy representations are entirely identical (Figure 1.1), the distinction of bonds and intermolecular forces is not artificial. Thus, chemical bonding obeys magic numbers (electron pairs, octets, Hückel’s 4n+2 numbers, etc.), and defines chemical identity, while the intermolecular interaction, as a creator of aggregates, is an extensive property that does not obey magic numbers and has much lesser selectivity. In other words, bonds are formed to satisfy the atomic valences, while intermolecular interactions are residual interactions formed between species, which have satisfied their formal valences. Consequently, diatomic bonds are generally stronger than the respective diatomic intermolecular interactions, and as such, ‘bonds’ make molecules, while ‘intermolecular interactions’ create aggregates of molecules.
Figure 1.1 One dimensional energy representation of stabilizing interactions, along some general proximity coordinate R, which can be a distance between two bonded atoms or a generalized proximity coordinate between molecules or aggregates. Re defines the equilibrium structure. De is the dissociation energy.
It is deemed necessary therefore to conserve this distinction in view of the current vogues of calling every stabilizing interaction, even as in He2, a bond; a tendency that has already been criticized.3 This distinction is also didactic, playing an important role in the comprehension and teaching processes of the structure of chemistry. In fact, it is this distinction that has enabled the development of chemistry as a molecular science.4
In many ways, bonding and intermolecular interaction is a field in a midst of a great renaissance.5,6 There are new interesting theoretical approaches to probe the origins of bonds, many novel bonding motifs,7,8 and experimental studies that describe imaging of bonds being broken and remade using atomic force microscopy.9 The bond is becoming again a central intellectual arena, and one can even find allusions to the bond as an elementary particle of chemistry, the so-called “bondon”.10 Likewise, the interest in intermolecular interactions has moved to new frontiers with bonding motifs such as “halogen bonds” and CH⋯HC interactions, and methods to calculate and analyze them.11–15 There exist also new chemical bonds that are supported by dispersion interaction, such as very long C–C bonds.16 There are even some claims of visualizing hydrogen bonds and of bond breakage and remaking. This “return of the bond and intermolecular interactions” to the central arena of chemistry marks the revitalization of chemistry as an intellectual science.
This chapter tries to convey the feeling of the author that the chemical bond and intermolecular interactions are now frontier domains in chemistry. And, by the nature of this goal, the chapter does not present an exhaustive treatise of bonding but rather a bird’s eye view with examples, which reflect personal tastes of the author and some reliance of science writers like Ball and Ritter.5,6 As such, the chapter adopts in places an essay-like style that addresses the target audience, rather than bringing a rigorous equations-laden exposition that would appear in source material.
The chapter starts with a short historical perspective of the evolution of the bond concept from the times of alchemy, through the constitutional revolution by Lavoisier, Cannizzaro, and Dalton, to the electronic structure revolution by Lewis, and the second revolution of quantum mechanics, where valence bond theory (VBT) and molecular orbital theory (MOT) were developed and imported to mainstream chemistry. It continues with the energy perspective of the origins of bonding, and then shows the unity of outlooks on bonding, emerging from VBT, MOT and density functional theory (DFT). The narrative goes on with very brief description of new bonding motifs, and then it switches to intermolecular interactions.