![]()
CHAPTER 1
Co-crystals: Introduction and Scope
CHRISTER B. AAKERÖY* AND ABHIJEET S. SINHA
Department of Chemistry, Kansas State University, Manhattan, Kansas 66506, USA
*Email:
[email protected]1.1 Rationale and Scope
The structure and chemical composition of a molecule are responsible for a wide range of properties, especially in solution and in the gas-phase. However, the three-dimensional orientation and organization of molecules in a crystalline lattice determine many bulk properties of a substance, e.g. density, solubility, thermal stability, optical properties, hygroscopicity, and mechanical strength.1 Consequently, if we are seeking to fine-tune or ‘dial-in’ physical properties through a bottom-up approach, then we need to acquire control over the metrics and topologies of the structural landscape that is inhabited by a particular target molecule. Unfortunately, this presents considerable challenges because molecular recognition events,2 which provide the driving force behind any supramolecular assembly,3 are the result of a delicate balancing act between relatively weak and reversible non-covalent interactions. Thankfully, supramolecular and structural chemistry have given us invaluable information about the inherent directionality and selectivity of many non-covalent interactions, and through the use of co-crystals we may be able to harness this knowledge in the preparation of materials with tailored physical properties.
One of the reasons why co-crystals offer unique opportunities for deliberate adjustments of bulk properties is that within a series of co-crystals of a target molecule, it may be possible to make modular changes to the crystalline framework that contains the ‘active’ molecules. This, in turn, may allow us to make incremental (and predictable!) changes to the physical properties and performance of a substance without having to alter any of the molecular properties of the target. The outcome from many systematic studies on synthesis and characterization of co-crystals are already having a positive impact on the deliberate design of new functional solids and materials. In addition, co-crystallization offers unique opportunities for probing intermolecular competitions, which in turn may help improve our central understanding of how fundamental laws of physics manifest themselves in crystalline materials. The inexorable (but not always obvious) connections between structure, crystal morphology and bulk properties means that with the ability to change specific aspects of the solid form of a compound comes new and unique prospects for materials design that could undoubtedly be highly beneficial to manufacturers and consumers alike. Finally, in order to continue to make progress in this area, we will need expertise from organic, inorganic, physical, materials, and theoretical chemists. This will require and facilitate unique interfaces of experimental and theoretical tools, and more extensive and open collaborations between academia and industry.
1.2 Covalent Versus Non-covalent Synthesis
Chemical transformations that proceed through bond-breaking and bond-making events involving covalent bonds have been studied and refined for well over 150 years.4 A wealth of information has been acquired and as a result we now have a thorough understanding of the interplay between molecular structure, chemical reactivity, and reaction pathways. Consequently, organic synthesis has had an unparalleled impact on all aspects of modern society.
The design of synthetic pathways for co-crystal synthesis, and the practical implementation and optimization of such protocols, are undoubtedly part of chemical synthesis, and it is therefore inevitable that we occasionally try to draw comparisons between covalent and non-covalent synthesis. It is well known that organic synthetic chemists can guide site-specific reactivity via substituent-guided modulation of the electronic environment of a molecule. For example, the methyl group of toluene activates the benzene ring towards electrophilic aromatic substitution at ortho and para positions, whereas the nitro group deactivates the benzene ring in nitrobenzene and substitution occurs meta to the nitro group. However, in controlling and predicting the supramolecular assembly of molecules, the focus is on site-specific interactions and reactivity of an intermolecular nature. Given a system containing two strong, competing intermolecular interactions, can we tip the balance in favor of one interaction in order to control the resulting 3-D structure? Can crystal engineers use tools forged by synthetic organic chemists to construct supramolecular architectures? More specifically, can we use substituent effects to ‘switch’ intermolecular interactions on and off by altering the electronic environment of individual molecules? A grand challenge for crystal engineering is to develop synthetic protocols that are robust, versatile, and transferable and that, ultimately, can approach the level of reliability that name reactions offer to conventional chemical synthesis. At the same time, it is also important to set realistic goals and targets. The majority of chemical reactions tend to work on a narrow set of substrates, under specific reactions conditions or with tailor-made catalysts, and they have invariably been developed and optimized via lengthy procedures. Also, chemical synthesis can be achieved in multi-step reactions where it is possible to isolate, characterize, and purify intermediates and products along the way, but supramolecular synthesis is generally restricted to one-pot reactions. Hence, when measuring the relative success of a supramolecular synthetic protocol, it is reasonable to recall that, in chemical synthesis, which is correctly viewed as a mature science, many chemical reactions still fail to deliver the desired product in high yields. Thus, even though there are close similarities between covalent and non-covalent synthesis, Scheme 1.1, practitioners of the latter variety often face unique challenges.
Scheme 1.1 Covalent vs. supramolecular synthesis.
1.3 History
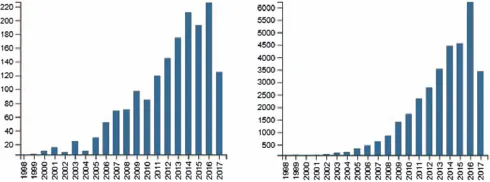
There is general agreement that the history of co-crystals dates back to 1844 when Friedrich Wöhler combined solutions of quinone and hydroquinone resulting in a green solid, quinhydrone, which he proposed contained both reactants in 1 : 1 stoichiometry.5 For several decades, there was disagreement about the exact nature of the chemical composition of Wöhler’s product and, remarkably, it took over a century before the composition and structure of this crystalline material could be unambiguously verified through single-crystal X-ray diffraction.6,7 Interestingly, a triclinic polymorph of quinhydrone was reported as late as in 1968.8 A close examination of the literature from the late 19th and early 20th centuries reveals the presence of literally hundreds of co-crystals (although they would invariably appear under different names) and Stahly has published an excellent comprehensive summary of co-crystals reported prior to the year 2000.9 Numerous review articles have also provided extensive coverage of the field as it has developed since the beginning of the new millennium.10 The focus on co-crystals as a readily identifiable research owes a lot to Etter’s groundbreaking work in the late 1980s and early 1990s,11,12 as well as to Desiraju’s seminal book on ‘crystal engineering’.13 The way that the field has developed since the early 1990s is readily illustrated by an examination of the relevant literature, Scheme 1.2.
Scheme 1.2 Results from a SciFinder topics search on ‘co-crystals’ (July 2017). Total number of publications (left) and total number of citations...