
- 586 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Photopolymerisation Initiating Systems
About this book
Photoinitiating systems play a key role in the starting point of a polymerization reaction under exposure to a UV or a visible light. The number of publications discussing photoinitiating systems for polymerization has seen a significant growth in recent years and this book provides an update on their latest research developments.
The book covers different types of photoinitiating systems including UV radical photoinitiators, long wavelength sensitive radical photoinitiators, cationic photoinitiators and water soluble photoinitiators as well as a chapter on how to design novel photoinitiators. The book then focusses on the applications of the photoinitiators from nanoparticles and materials to ionic liquids and solar cells.
Edited by leading names in the field, the book is suitable for postgraduate students and researchers in academia and industry interested in polymer chemistry, organic chemistry, materials science and the applications of the materials.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
*Email: [email protected]; [email protected]
1.1 Introduction
| Thioxanthone derivative | λmax (nm) | ε at λmax (M−1 cm−1) |
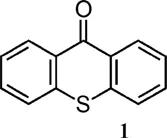 | 380 (benzene)6 381 (DMF)5 | 6600 (benzene)6 6328 (DMF)5 |
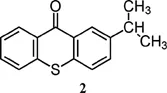 | 386 (benzene)6 | 6900 (benzene)6 |
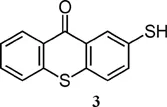 | 383 (THF)7 | 3857 (THF)7 |
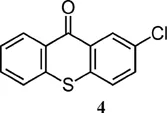 | 388 (benzene)6 | 6700 (benzene)6 |
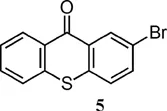 | 388 (DMF)5 | 4941 (DMF)5 |
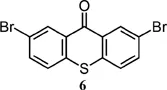 | 396 (DMF)5 | 5234 (DMF)5 |
 | 438 (THF)8 | 4470 (THF)8 |
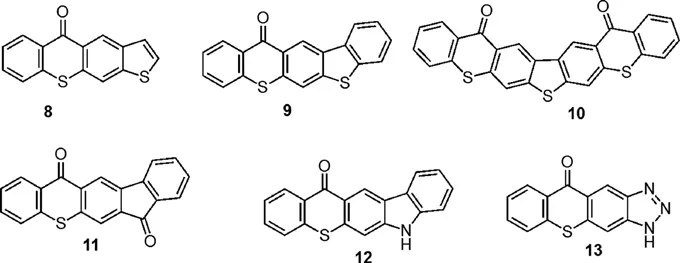
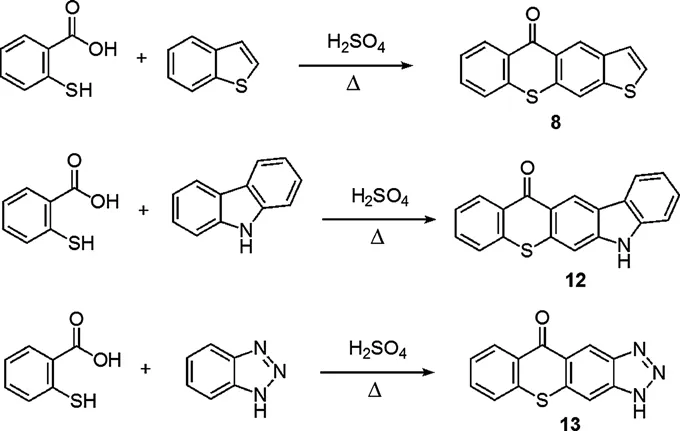
1.2 Photophysical Properties of Heterocyclic Extended Thioxanthones
Table of contents
- Cover
- Title
- Copyright Page
- Preface
- Contents
- Chapter 1 Thioxanthone Photoinitiators with Heterocyclic Extended Chromophores
- Chapter 2 Long-wavelength-sensitive Radical Photoinitiators
- Chapter 3 Cationic Photoinitiators
- Chapter 4 Monomeric and Polymeric Photoinitiators
- Chapter 5 Photoinitiators for Blue to Red LED Exposures
- Chapter 6 How to Design Novel Photoinitiators for Blue Light
- Chapter 7 Photocatalysts as Photoinitiators
- Chapter 8 Controlled Reversible Deactivation Radical Photopolymerization
- Chapter 9 Photosynthesis of Polymeric Particles
- Chapter 10 Photoinitiators in Ionic Liquids
- Chapter 11 Photoinitiators in Dentistry: Challenges and Advances
- Chapter 12 ZnO Nanoparticle-based Photoinitiators
- Chapter 13 Water-soluble Photoinitiators: Present and Future
- Chapter 14 NIR Light for Initiation of Photopolymerization
- Chapter 15 D–π–A-type Sulfonium Salt Photoinitiators for Photopolymerizations Under Near-UV and Visible Light-emitting Diodes
- Chapter 16 Photopolymers for Third-generation Solar Cells
- Chapter 17 Photopolymerization of Amphiphilic Molecule Self-assemblies
- Chapter 18 Emulsion Photopolymerization
- Conclusion
- Subject Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app