![]()
Chapter 1
Introduction
The statement that biocatalysis is of great importance sounds trivial in face of the fact that biocatalysis is the prerequisite for any life at all. Application of biocatalysis has a long tradition if the unwitting employment of the underlying processes in ancient times is included, e.g. fermentation in connection with beer brewing or the baking of bread. In present days, the emerging research results in life science, and especially in biocatalysis, increasingly influence all areas of daily life. Modern developments in medicine, pharmacy, nutritional products, analytics, environmental technology, and others are inconceivable without the innovative research results in biocatalysis.
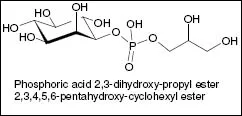
Millions of years of evolution have generated an unimaginable multiplicity of organisms. Biocatalysts regulate and control all metabolic reactions in microorganisms, plants and animals in a very selective way and make the necessary high reaction rates possible. Reaction conditions are either mild or adapted to the special requirements of the milieu an organism develops its activities in. Microorganisms have been found to live under unusual environmental conditions, e.g. disused chemical plants; they adapted to their surroundings by developing an enzyme equipment that enables them to degrade the chemicals they discovered. So-called barophiles populate deep sea habitats where they grow at temperatures just above 0°C, and at a pressure of more than 1000 bar but perish when temperature increases and the pressure decreases; psychrophiles metabolize at even lower temperatures, e.g. under the conditions of the Siberian permafrost. Of even more biotechnological relevance are extremophiles – microorganisms existing in so-called ecological niches, first found in the hot springs of the Yellow Stone National Park about 50 years ago. They produce enzymes that function at temperatures up to 130°C and extreme pH-values. Such robust enzymes are of great interest for various biotechnological processes. Interestingly, not all enzymes isolated from hyperthermophiles are particularly heat stable. These organisms obviously developed alternative strategies to survive under such conditions as e.g. to synthesize low-molecular mass metabolites exerting in vivo a stabilizing effect via an interaction with proteins by a mechanism, not fully understood so far. To these so-called compatible solutes belong α-and β-glutamate (accumulated for osmoadaption), di-myo-inositol phosphate and 1-glyceryl1-myo-inositol phosphate(opposite figure), recently discovered in the hyperther-mophilic bacterium Aquifex pyrophilus in response to both osmotic and heat stresses (Lamosa et al., 2006). The interesting question whether these solutes can be used to stabilize other isolated enzymes still needs to be elucidated. Altogether, there should exist innumerable different enzymes (most of them not detected so far) to catalyse most types of chemical reactions. In view of these facts the importance of biocatalysis lies in its enormous potential for application becoming more and more evident in recent years as new sensational research results emerge.
Not long ago enzymes had to be employed as provided by Nature. However, as new biological and molecular tools as mutagenesis or recombinant technologies became available, it was possible to influence the properties of biocatalysts with respect to catalytic activity, selectivity, and stability. High stability of a biocatalyst under process conditions is a prerequisite for its economic application in the industrial production of high-value fine chemicals as well as bulk compounds, in order to create a competitive alternative to traditional chemical procedures.
Parallel to the development of knowledge in this field, new terms as proteomics, genomics designer bugs, etc. have emerged, some of which were not to be found in relevant textbooks or dictionaries 10 or 15 years ago. Structural genomics stand for all methods by which highly resolved structural information of all proteins coded by a genom are gained. The aim of proteomics is to identify and characterize all proteins within e.g. a cell, together with an analysis of their interactions under varying conditions, making proteomics rather complex. With these and other methods and strategies metabolic pathways of organisms can be elucidated in detail; corresponding results establish to an increasing degree the basis for the optimisation of bioprocesses or for a targeted change of the catalytic profile of a microorganism by genetic modification.
Nowadays, research in natural sciences is shaped considerably by results in life sciences and material sciences, with nanosciences taking a prominent role in the latter field. So-called smart materials have been found in recent years, responding to parameters of their surroundings, i.e. temperature, pH-value, or salt concentrations. They have been successfully employed in the controlled release of pharmaceutically active compounds as well as in the field of immobilized biocatalysts. The immobilization of enzymes onto the surface of nano-structured materials with the aim to produce a biosensor is an example for ‘applied nanobiotechnology’. All these developments demonstrate the multidisciplinary character of the research in biocatalysis. Emil Fischer was probably first in formulating this in a visionary way already in 1907 in his Faraday Lecture to the Chemical Society about ‘Synthetic Chemistry’ and its Relation to Biology when he said: “….the separation of chemistry from biology was necessary while experimental methods and theories were being developed. Now that our science is provided with a powerful armoury of analytical and synthetic weapons, chemistry can once again renew the alliance with biology, not only for the advantage of biology but also for the glory of chemistry”. However, it took approximately seven further decades until the cooperation between chemists and biologists (and scientists of other research fields) by and by became a matter of course.
1.1 Advantages and Disadvantages of Biocatalysts
Nature has created excellent catalysts by evolution over millions of years. They are mostly proteins (enzymes or catalytic antibodies) but also nucleic acids with catalytic properties similar to those of enzymes detected in the early 80s. Up to now, of these natural catalysts only enzymes are used in applied biocatalysis.
Enzymes catalyze chemical reactions (and energetic transformations) in a single cell or in a whole organism, essential for survival and reproduction. A biocatalyst may either be the complete cell itself, employed in a viable, non-viable, growing or non-growing state, or an individual enzyme. The properties of enzymes, compared with catalysts normally used in chemical processes, are remarkable. Enzymes exist for nearly all reactions known in Organic Chemistry. Characteristic for each catalyst, they increase the rate at which equilibrium is attained without effecting the equilibrium constant by providing an alternative reaction path with a lower activation energy than the one of the corresponding uncatalyzed reaction. What is spectacular is the degree of rate acceleration.
Table 1.1: Some of the main advantages and disadvantages of biocatalysts against the background of their possible application in biotransformations on laboratory or industrial scale.
| Advantages | | Disadvantages |
- Very efficient catalysis of most known chemical reactions
- High regio-and stereoselectivity
- Mild reaction conditions and thus low energy consumption
- Amount of byproducts is low
- They are biodegradable
- Preparation on large scale is possible through fermentation (microbial enzymes)
- Reuse is possible (immobilization)
- They can be designed to a certain extent
- They are non-toxic if correctly applied
| | - Protein molecules are rather instable in aqueous media
- They may be inactivated by
- higher temperatures
- extreme pH-values
- higher salt concentrations
- (polar) organic solvents
- Inactivation may further occur through inhibition by
- substrate
- product
- metal ions
- inhibitors
- Many enzymes are cofactor-dependent
- Allergic reactions possible
|
An impressive example is given by the enzyme catalase that catalyzes the decomposition of hydrogen peroxide. From the activation energies for the uncatalyzed and the catalyzed reaction, i.e. 75 kJ/mol and 8 kJ/mol, respectively, results a factor of rate enhancement of about 1015. This individual value lies at the upper limit, usually such factors for enzyme-catalyzed reactions are between 1010 and 1012.
Apart from this, enormous rate acceleration allows reactions to proceed under physiological conditions in a split second that would take ages to reach equilibrium without a catalyst; enzyme-catalyzed reactions are often highly substrate-specific without forming byproducts, a consequence of their regiospecificity. Furthermore, due to the fact that enzymes are asymmetric molecules, they have the ability to precisely differentiate between stereoisomers, resulting in the formation of chiral products whereas chemical synthesis often leads to racemic mixtures. All these properties make enzymes interesting candidates as catalysts for industrial processes; however, as always, enzymes also have disadvantages limiting their use as catalysts in chemical reactions. Due to their flexible molecular structure they are rather instable in aqueous media, and are inactivated by higher reaction temperatures, or extreme pH values and salt concentrations. They may be inhibited by their own substrate, by the react...