Helmholtz-Institute for Pharmaceutical Research Saarland, Department Drug Design & Optimization, Campus E81, Saarbrücken 66123, Germany
1.1 Of Peptides and Proteins (and Small Molecules)
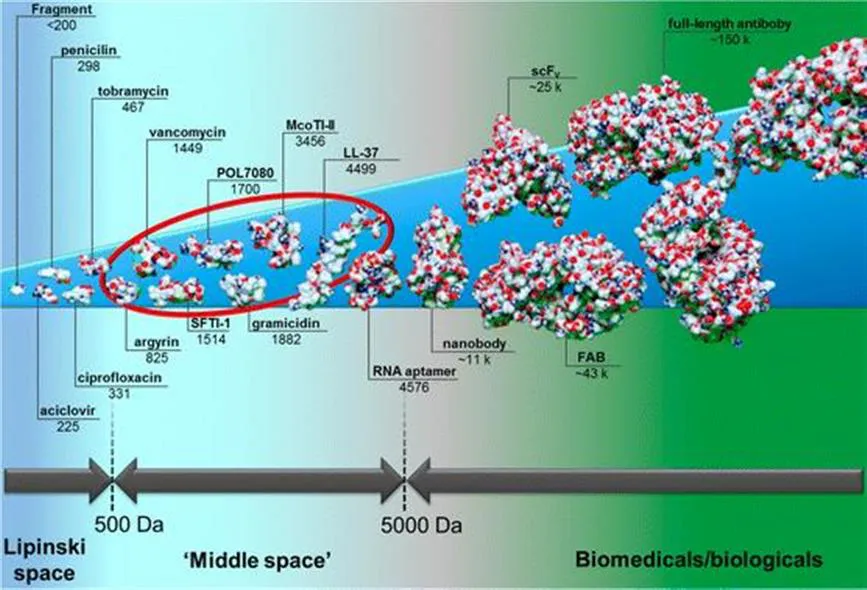
When we compare the Ancient Greek etymologies of ‘protein’ and ‘peptide’ it becomes evident that the former of these very closely related biomolecules is associated with rather positive attributes, as its name derives from proteios (πρωτεῖoς) meaning ″the first quality”.1 Peptides, on the other hand, seem to be considered a mere rudiment of their bigger ancestors, as they are referred to with a terminus derived from peptós (πεπτóς), meaning “digested” or “cooked”.2 But what is the decisive characteristic that defines the ‘quality’ of proteins, which peptides supposedly lack? Both biopolymers usually consist of a linear sequence of amide-linked building blocks, which in most cases are a selection from the standard repertoire of the 20 proteinogenic amino acids. Usually, amino acid sequences shorter than 50 residues are considered as peptides and, thus, these oligomers reside in the so-called ‘middle space’ (see Figure 1.1).3,4
Figure 1.1 The scale of pharmaceutical agents divided into small molecules (Lipinski space), the ‘middle space’, and the biomedicals/biologicals illustrated by selected anti-infective agents and other examples. The range commonly occupied by (cyclic) peptides is highlighted with a red elliptical shape. From small to large: a fragment-sized compound,5 acyclovir, penicillin, ciprofloxacin, tobramycin, argyrin,6 vancomycin,7 sunflower-trypsin inhibitor-1 (SFTI-1),8 POL7080,9 gramicidin,10Momordica cochinchinensis trypsin inhibitor-II (McoTI-II),11 human cathelicidin LL-37,12 an RNA aptamer,13 a vNAR antibody fragment as a representative for ‘nanobodies’,14 a single-chain variable fragment (scFv),15 a FAB-fragment,16 and a full-length IgG antibody.17 The corresponding molecular weights are given in Da.
This term has been coined to refer to molecules with a molecular weight between 500 and 5000 Da (or maybe only approximately 3000 Da). Noteworthily, most of the active principles in pharmaceutical drugs belong either to small molecules or biomedicals.18 The former usually obey the well-known rules-of-five set up by Lipinski, while the most prominent members of the latter are immunoglobulins or derivatives thereof.19 Members of the ‘middle space’ are still comparably rarely found in pharmaceuticals that are in clinical use and small molecular drugs make up about 90% of the pharmaceutical market.20,21 However, their share is continuously growing.20
So, size as a molecular descriptor might give us a hint toward the ‘special quality’ of proteins. However, we know of so-called ‘mini-proteins’ (e.g. McoTI, see Figure 1.1), which can be smaller than 40 residues and exert fascinating biological functions, nonetheless.22,23 Hence, certainly, the line between peptides and proteins is fuzzy and not so clear cut as one might think. Which brings us back to the initial question: ‘What is the unique quality of proteins, then?’
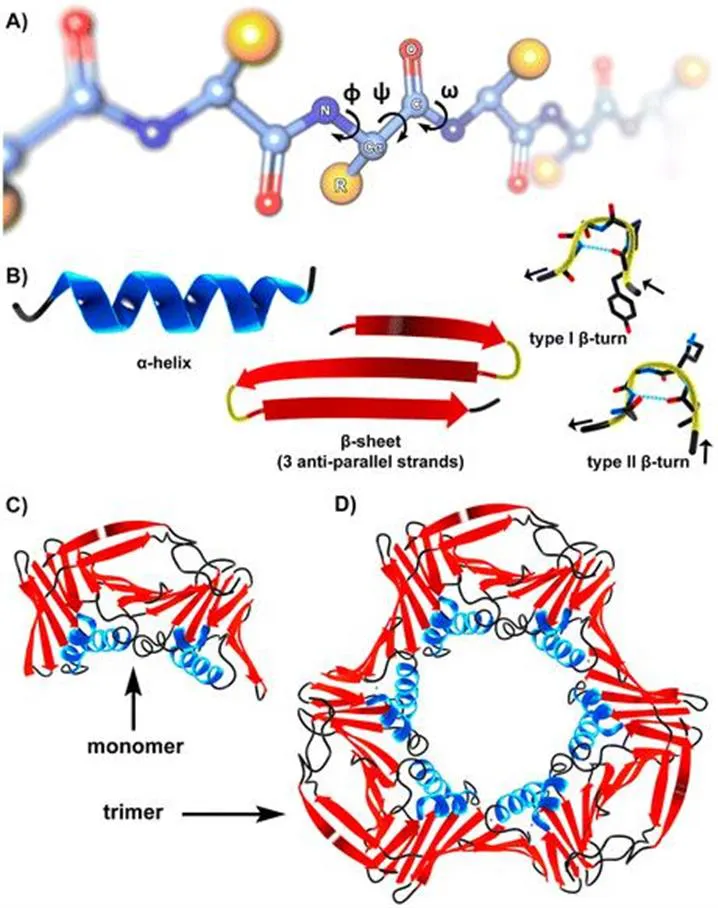
One or maybe THE extraordinary characteristic of protein chains is their ability to adopt precise spatial arrangements of each individual rotatable bond within their backbone. Only when the huge array of so-called Φ, Ψ, and ω dihedral angles is assembled in the right way (see Figure 1.2A), is the protein folded into its correct three-dimensional structure.24 Partial motifs of protein folds (secondary structures) can also be present in peptides (Figure 1.2B).25 The most prominent secondary structures are of course α-helices and β-sheets. Aside from larger loops, which are usually not well defined, β-turns should be highlighted as important structural motifs connecting, for example, the separate strands of a β-sheet.26 However, full tertiary or even quaternary assemblies are usually not found for peptides (Figure 1.2C and D). The reason for this is that a short peptide chain offers fewer opportunities for structure-defining intramolecular interactions, which are needed to render a desired three-dimensional structure thermodynamically favorable. Instead, linear peptides are generally quite flexible and do not adopt an unambiguous geometry.27 As the structure dictates the function of a biomolecule, we may now have approximated the answer to the question raised above: linear peptides often lack a well-defined structure, while proteins exert their various activities through folding into stable three-dimensional assemblies.28
Figure 1.2 (A) A generic peptide chain illustrating the Φ, Ψ, and ω dihedral angles at one amino acid residue. The consecutive order of amino acids in the polypeptide chain is called the primary structure. (B) Schematic depictions of the most common peptide secondary structures: α-helix, β-sheet (3 strands), and type I as well as type II β-turns. (C) and (D) Tertiary and quaternary structure illustrated for the example of proliferating-cell-nuclear-antigen (PCNA).31
This short repetition of textbook knowledge above instantly raises another question: ‘Is there a way to fix a well-defined structure within a peptidic biomolecule?’ Well, the title of the book you hold in your hands readily reveals the answer: Yes! – and it can be achieved through introducing cyclic or macrocyclic motifs! Nature and scientists alike make use of these conformational constraints, which drastically reduce the degree of rotational freedom within the backbone, thereby orienting the side chains in favorable directions and, thus, tethering fascinating biological activities and other favorable properties into otherwise inactive peptide sequences.29,30
1.2 Conformational Constraints
Restricting the conformational freedom of a peptide can be achieved by different means.29 In addition to the just-mentioned macrocyclization strategy, available torsional angles can be reduced through bulky amino acid side chains, where only some of the possible dihedral combinations are allowed at the corresponding and neighboring residues due to steric hindrance.32 Furthermore, the presence of a proline readily fixes the Φ dihedral at this residue as a consequence of the covalent linkage of the alkyl side chain to the α-amine. The ω dihedral, which is commonly referred to as the peptide bond, can be cemented into each of the two possible conformations through biomimetic exchange, for example by the use of 1,2,3-triazoles or other heteropentacycles.33 However, introducing a cycle/macrocycle is the strategy that has the most prominent impact on the overall conformational freedom effecting a multitude of residues and not only some selected positions within the peptide chain.29
There are three straightforward concepts to achieve a looped structure within a peptide:
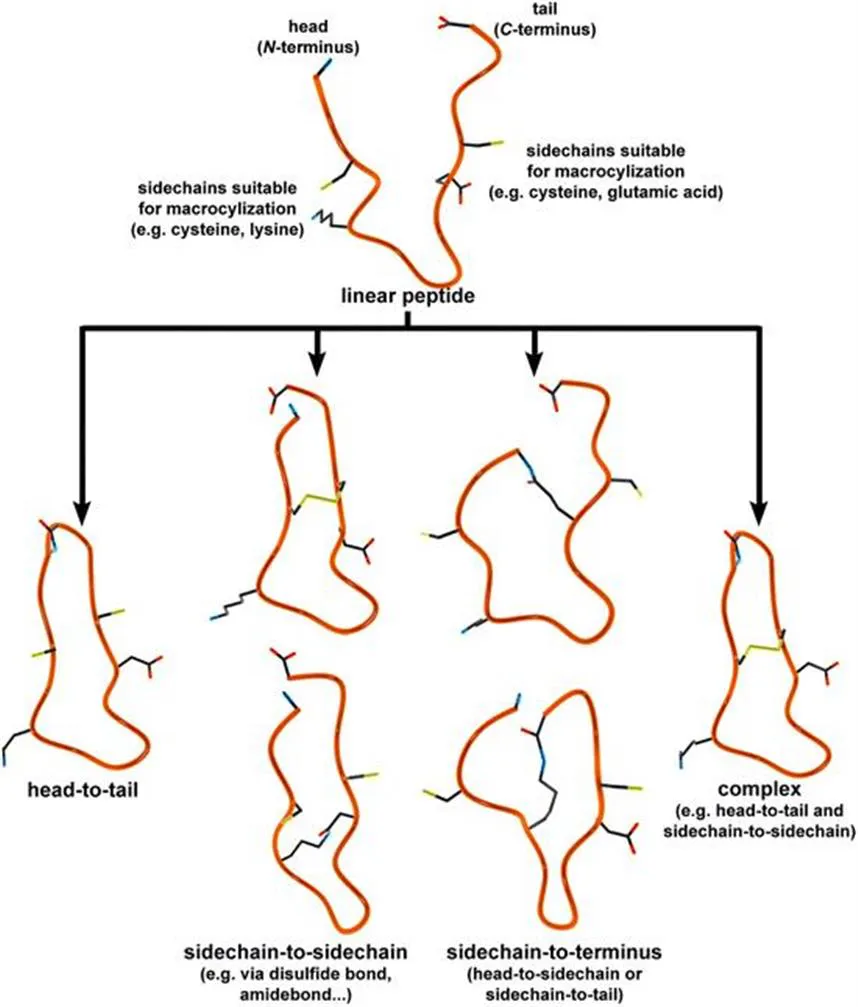
Head-to-tail-, side chain-to-side chain-, and side chain-to-terminus-cyclization.34,35 Multiple loops are also regularly found in peptides, e.g. a combination of a head-to-tail- and a side chain-to-side chain-macrocycle. If the looped amino acids are only connected via amide bonds, this compound is called ‘homodetic’.35 In cases where any other linkage is involved (e.g. disulfide or depsipeptide bonds), it can be referred to as a ‘heterodetic’ peptide.35 According to the IUPAC, a macrocycle consists of at least twelve atoms. Hence the simplest homodetic macrocyclic peptide is a covalent circuit built up of at least four residues. However, smaller (non-macro-)cyclic motifs also occur containing only a limited number of constrained bonds. Figure 1.3 depicts some of the most simple and common motifs.34 Comparing these structures, it immediately becomes clear that each individual mode of cyclization can result in a completely different geometry of the looped compound.
Figure 1.3 Common modes of macrocyclization shown for the example of a generic peptide chain containing two cysteines, a lysine, and a glutamic acid. All other residues are not shown. The peptide backbone is given in orange, carbon in black, nitrogen in blue, oxygen in red, and sulfur in yellow.
Noteworthily, far more complex patterns have been observed in peptidic natural products including, for example, thioether linkages commonly found in lanthipeptides.36 As described for proline, the backbone amide nitrogen can also be used as an anchor point for building a covalent ring...