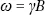ANTHONY C. DONA†
MRC-NIHR National Phenome Centre, Department of Surgery & Cancer, Imperial College of London, South Kensington Campus SW7 2AZ, United Kingdom
1.1 History of NMR Hardware Development
The basic function of an NMR spectrometer is to measure the frequency of the resonance of a given nucleus. After the first decade of discovery (1946–1955), the basic NMR relationship was established (eqn (1.1)), which suggests the resonance frequency of a nucleus (ω) to the magnetogyric ratio (γ, specific to nucleus type) and the external magnetic field (B).
At this stage it was thought that the frequency of a nucleus depended entirely on the strength of the magnetic field in which it was placed. In 1949–1950, however, observations from 19F and 31P showed variations in frequency between the two types of nucleus beyond the still rather large experimental error. Furthermore, after developments in the stability and homogeneity of the magnetic field, two separate resonances where observed from the hydrogen atoms in ethanol1 and separately for nitrogen atoms in different chemical environments. This phenomenon soon became known as ‘chemical shift’ as the frequency of a resonating nucleus is largely dependent on the local chemical environment surrounding the nucleus itself. Further improvements in the resolution allowed separate resonance lines to be observed from a single chemical resonance, allowing the discovery of the concepts of indirect spin–spin coupling or scalar coupling.2 In the late 1950s and the into the 1960s the strength of the field increased to 100 MHz and commercial instruments were developed by Varian that maintained a constant relationship between the magnetic field and the radiofrequency (RF) applied so spectra could be recorded at a known scan rate. In this time the first 13C spectrum was recorded,3 made difficult at the time by the low natural abundance of 13C atoms (1.1%). Carbon spectroscopy really became popular with the advent of double resonance, where two RF fields are applied to a sample simultaneously in order to measure one spin system while the other is perturbed. From double resonance, applications such as spin decoupling experiments and nuclear Overhauser effect (nOe) were introduced to aid studies of molecular conformation.4
In 1966, Ernst and Anderson published work5 showing that Fourier Induction Decay (FID) following a short RF pulse was all that was necessary to produce a spectrum measuring a range of frequencies. Additionally, minicomputers were being developed to interface directly with the spectrometer, allowing Fourier Transform NMR (FT-NMR). These hardware advances revolutionised NMR spectroscopy, supporting the enhancement of sensitivity (which was NMR's main disadvantage compared with other spectroscopy techniques) and enabling exploitation of time-dependent NMR phenomenon, namely relaxation.
Since these major advances in NMR hardware, many pulse sequences and applications have developed over the last 40 years, making NMR spectroscopy an incredibly versatile tool in chemical and biochemical research areas. Magnetic Resonance Imaging (MRI) was also made possible by imposing magnetic field gradients across a sample in vivo. Two-dimensional and eventually three-dimensional NMR imaging was made possible with wide bore magnets (wide enough to fit animals or humans through) and the measure of a frequency across a spatial gradient.6 Techniques are also currently being produced to integrate spectroscopy with imaging to obtain localised spectra in living creatures.
These days, the hardware available for modern-day NMR measurements allows for routine acquisitions with relative ease, in small (metabolite, organically synthesised) and large (protein) molecules, in either purified solutions (for molecular structure elucidation) or complex mixtures (for solution composition elucidation).
1.2 Components of NMR Hardware
1.2.1 Magnet
The NMR magnet itself is generally considered the most important part of the NMR spectrometer and is commonly the most expensive piece of equipment for a standard NMR laboratory. Before the advent of superconducting magnets, iron-core electromagnets had enabled field strengths of 2.35 T to be reached. At this stage iron saturates, so a great effort was required to develop a magnet that could be housed in a liquid helium Dewar to obtain the temperatures required to cool a superconducting solenoid. Today, commercial NMR magnets are generally superconducting and range in field strength from 6 to 23.5 T. Other physical chemistry laboratories are developing larger magnets, not intended for commercial distribution, with magnetic field strengths of up to 45 T.7 With increasing strength, not only does the size of the magnet need to increase but technologies need to be developed to break ground in new magnet generation. As magnets increase in size (and so magnetic field strength) their resolution and sensitivity of frequencies improves, but their cost also increases substantially, meaning larger magnets are much less accessible.
Superconducting wires are generally made from Nb3Sn or (NbTaTi)3Sn, which are wrapped hundreds of times into a coil making up a length of wire up to 100 km long. Few higher temperature superconducting materials are suitable for manufacturing in the quality and quantity required for NMR magnets, so very low temperature superconductors (∼10 K) are usually constructed. The wire has a rectangular cross section allowing maximum current density and therefore maximum magnetic field strength. This coil is kept inside a large Dewar containing liquid helium, keeping the coil at superconducting temperatures, which is in turn surrounded by a liquid nitrogen reservoir acting as a buffer between the room temperature air and the liquid helium. A significant property of the wire is the maximum critical current (Ic), which is a function of the operational temperature (T) and magnetic field (B). If the critical value is reached there is a transition in the wire from a superconductive to a resistive state, which in turn generates heat. The heat propagates rapidly through the coil prompting the energy store to be converted to heat, which induces the helium store to boil extremely rapidly. The loss of the superconductive state is known as a ‘quench’ and magnetic coils are developed to avoid quenches at all costs. Modern day magnets have an additional coil outside the main coil, which is used to contain the strong magnetic field by cancelling (shielding) the stray field, restricting it to a relatively small area.
The cryostat, which is the vessel surrounding the magnetic coil, must also be designed to be insensitive to variations in the environment and other disturbances such as helium evaporation rate, ambient temperature and pressure, and the cryogen levels inside the cryostat. New technologies in this area are focused around either using the complete enthalpy stored in the helium gas to enable further cooling of the system or otherwise recycling helium such that it is not lost to the atmosphere. These technologies are aimed at having the lowest possible helium consumption as it becomes a rarer and more expensive commodity.
NMR laboratories are often forced to compromise on magnetic field strength owing to available funding and space. Modern day magnets between 400 and 600 MHz are commonplace in metabolomics laboratories as they are now constructed to fit in a room without roof height modification. With modern day shielding, the footprint required for the magnetic field is not much larger than the magnet itself and so magnets of this size can essentially be lined up next to one another. Magnets of 600 MHz are produced much more readily than those of larger size and so the production cost is far cheaper and more reliable, making them the magnet size of choice for routine metabolomics studies. On the other hand, magnet sizes smaller than 400 MHz do not enable researchers to resolve important metabolite signatures in complex biofluids and so these are generally overlooked when purchasing a magnet for metabolomics purposes.
1.2.2 Shim Coils
Shim coils are a set of conducting coils used to adjust the homogeneity of a magnetic field. In the past, shimming (the process of optimising the homogeneity of the magnetic field) a magnet consisted of attaching thin metal shims in various positions around the permanent magnet. Coincidently, the term ‘shimming’ is used to describe the modern day process of homogenising the magnetic field across which the samples nuclei frequencies are measured. Modern high-resolution spectrometers alter the current in various conducting coils, which surround the external magnetic field, to alter it homogeneity.
When a spectrometer is installed, the local environment can disturb the magnetic field. Iron constructs in the walls and floor of the surrounding building disturb the homogeneity of the spectrometer's magnetic field. Therefore, upon installation spectrometers need to be roughly shimmed, after the initial activation of the magnetic field, with regards to the external environment. Once relative homogeneity is achieved, relatively minor changes in the magnetic field as a result of variations in the sample, the tube thickness and movement of ferromagnetic materials around the magnet are corrected before sample acquisition. These minor changes in the magnetic field are adjusted by changing the current in one or more (of up to 40) small shim coils with various gradients along all three spatial axes. In high-resolution spectrometers the magnetic field often demands homogeneity better than 1 part per billion in a tube, which is generally less than a millilitre in volume.
1.2.3 Sample Probe
The probe is the part of the spectrometer that does a lot of the physical experimental work and laboratories can often gain significant improvements in spectral quality by upgrading their spectrometer's probe. The probe contains the RF coils tuned to specific frequencies to excite particular nuclei and coils to detect the NMR signal. Pulsed Field Gradient coils, which allow for the application of field gradients, are also commonplace, allowing for the application of field-gradient pulses. The probe must also consist of the necessary hardware to measure and control the temperature of samples (a thermocouple device).
An important aspect of probe design is the size of the bore, which can alter to accommodate various sizes of tube. Small volume probes (3 mm) or nanotubes (1.7 mm) are able to give the greatest sensitivity, benefiting from the dramatic decrease in the diameter of the NMR detection coil. Some modern day probes are able to record good quality spectra from microlitre or even picolitre sample volumes. Nevertheless, in many cases larger volume bores (5 mm, 10 mm or wide bore) are necessary as the solubility or concentration of the sample is an issue. Larger volume bores are also recommended when measuring samples of higher viscosity or samples with micro-scale inhomogeneities (blood or emulsions), and are necessary when imaging small animals.
1.2.3.1 Radiofrequency Coils
Modern day probes are constructed with two coils to record NMR signal (known as observe coils). They are wrapped around one another such that there is an inner coil and an outer coil, allowing the probe to respond to different frequencies during the one experiment. This design also allows multiple nuclei to be excited during one pulse sequence. There are two main approaches to the design of these coils:
- - A ‘Broadband Observe Probe’ is constructed with the inner coil tuned to a broadband nucleus, and so is optimised for maximum sensitivity for nuclei at lower frequency (13C, 31P, etc.).
- - ‘Inverse Probes’ or ‘Indirect Detection Probes’ have the inner coil tuned to measure the frequency of 1H atoms and so get maximum sensitivity for proton experiments with much lower sensitivity when observing lower frequency nuclei.
1.2.3.2 Cryoprobes
Sensitivity of detection has dramatically improved with the advent of cryoprobes. These probes are significantly more expensive in design but ensure 2–4 times better sensitivity than standard probes. Cryoprobes have achieved the single largest jump in sensitivity enhancement by probe development in the last few decades by cryogenically cooling probe detector coils and preamplifier coils. As long as these coils, along with the tuning and matching circuits, are maintained at low temperatures the...