![]()
Chapter 1
Immunological Properties of Engineered
Nanomaterials: An Introduction
Marina A. Dobrovolskaia* and Scott E. McNeil
Nanotechnology Characterization Laboratory
Advanced Technology Program, SAIC-Frederick, Inc.
NCI-Frederick, Frederick, MD 21702, USA
Clear benefits of using engineered nanoparticles for biomedical applications are often challenged by concerns about the safety of these materials. Since the main job of the immune system is to efficiently detect and eliminate foreign materials from the body, nanoparticle effects on and interaction with various components of the immune system are active areas of research in current bionanotechnology and nanomedicine. Nanoparticles can be engineered to either avoid immune recognition or to specifi cally interact with the immune system. Below, we will provide a top level overview of the current state of science in the area of nanoimmunotoxicology, highlight common challenges associated with research of immunological reactivity of engineered nanomaterials, identify current gaps in our understanding of nanoparticle interaction with components of the immune system, and introduce other chapters in this book.
1. Introduction
Nanoparticle interaction with components of the immune system may produce different outcomes: 1) it may inhibit or suppress the immune system (immunosuppression) or 2) it may stimulate or enhance immune function (immunostimulation). Both immunostimulation and immunosuppression can be beneficial or detrimental depending on the intended use of an engineered
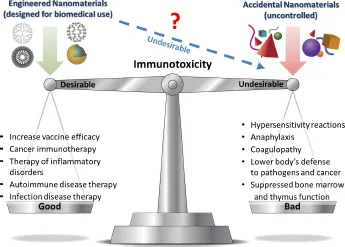
Figure 1. Nanoparticle immunotoxicity. Immunotoxicity of nanomaterials can be beneficial or detrimental depending on its desirability. Engineered nanoparticles can be designed to specifically interact or avoid interaction with the immune system. In contrast, accidental nanomaterials are more reactive and their interactions with the immune system are uncontrolled. Undesirable interactions between engineered nanomaterials and the immune system are the subject of basic research and preclinical studies.
nanomaterial (Figure 1). When immunostimulation is desired, it may aid in vaccine efficacy and therapy of cancer, while unwanted immunostimulation may lead to complications including hypersensitivity reactions, anaphylaxis, and thrombosis.1 Likewise, desirable immunosuppression is beneficial for the treatment of inflammatory disorders and autoimmune diseases, improvement of transplant acceptance, and prevention of allergic reactions, while undesirable immunosuppression may lower the body's response to infected and malignant cells, and lower bone marrow and thymus functions.1 It is now generally accepted that nanoparticle physicochemical properties such as size, charge, hydrophobicity, and presence of targeting moieties determine their interaction with the components of the immune system and immunotoxicity.2
Nanoparticles can be immunotoxic, however, so far no new, i.e., specific to nano, immunotoxicity has been reported. This is why it is currently accepted that immunotoxicity of engineered nanomaterials can be effectively assessed using the current portfolio of methods established for small and macromole-cules.3 Reviews of available data suggest that engineered nanomaterials are intrinsically no more immunotoxic than traditional drugs currently in use. Moreover, the incorporation of conventional pharmaceutics into nanotechnology-based platforms helps to decrease immunotoxicity of these traditional formulations. For example, benefits of using recombinant tumor necrosis factor-alpha (TNF-α) for cancer therapy were halted by systemic toxicity of this protein — fever and hypotension were dose-limiting adverse reactions associated with overt immunostimulation caused by this cytokine. The maximum tolerated dose (MTD) of this protein established in phase I clinical trials was 261 mg/m2 after slow (over 24 h) continuous intravenous (i.v.) infusion.4 Recently, the same protein was covalently attached to the surface of polyethylene glycol (PEG)ylated colloidal gold nanoparticles (Cyt6091 or Aurimmune™) and assessed in clinical trials. It has been reported that PEG– gold–TNF formulation was well tolerated by cancer patients when administered by bolus i.v. injection at 600 mg/m2 without reaching the MTD.5 Another two examples are cytotoxic oncology drugs paclitaxel and doxoru-bicin. The clinical application of the traditional formulation of the anticancer drug paclitaxel (Taxol®) is associated with severe hypersensitivity reactions even when this formulation is administered slowly and patients are premedicated with immunosuppressive agents, while nanoparticle formulation of the same drug (Abraxane® ) could be administered as a fast infusion and did not require premedication.6 Likewise, the clinical use of the traditional formulation of another anticancer agent doxorubicin (Adriamycin® ) is limited, among other side effects by disseminated intravascular coagulation (DIC), while incorporation of this small molecule drug into PEGylated nanoliposomes eliminates this toxicity.7
Although these examples are very encouraging, we have to keep in mind that each nanoparticle is unique and a positive experience with one formulation does not guarantee similar success with another, i.e., each nanoformulation needs a separate set of studies to understand its safety. The “shifting” of a drug's toxicity from one target organ to another due to the change in biodistribution caused by a nanocarrier is an important point to think about when evaluating nanoformulations. For example, nephrotoxicity is a common dose-limiting toxicity of the anticancer drug cisplatin.8 When cisplatin is incorporated onto the nanotechnology platform, it may follow the nanocarrier; if the nanocarrier is distributed to another organ, e.g., spleen or liver, the toxicity associated with cisplatin may “relocate” to these organs. This is why thorough pharmacokinetic (PK) studies assessing the distribution of both the drug and the nanocarrier are needed to identify potential changes in the drug's biodis-tribution and new site(s) of undesirable toxicity caused by this change.9 Avoiding the “double-hit” scenario is another important point to consider when designing nanoformulations with the aim of reducing toxicity of the legacy drug. For instance, if a nanoformulation is considered for the delivery of oligonucleotides (ODNs) for which complement activation-mediated pseu-doallergy (CARPA) is a common dose-limiting toxicity,10 it is better to consider a nanocarrier which is known not to cause CARPA. In this example, a nanocarrier in a form of PEGylated liposomes does not appear to be an optimal carrier choice for ODNs, because CARPA is a very common side effect of PEGylated liposomes.11 Of course, all of these examples are generalized and should not be used to discourage the development of an individual nanoformulation.
During recent years of research investigating the immunocompatibility of engineered nanomaterials, several common types of immunotoxicity have been identified. They are cytokine-mediated reactions, complement activation, coagulopathy (specifically DIC-like reactions), and the exaggeration of endotoxin responses. Among the common problems experienced in early preclinical testing, endotoxin detection and removal, nanoparticle interference with traditional in vitro assays, the lack of standard toxic reference materials, and the need for modified versions of traditional in vivo tests are on top of the list. Seven years ago, the entry of key words “nanoparticles” and “immune system” or “ immunotoxicity” into the PubMed database generated no results. Now, entering these words brings over 2,000 hits. A common problem limiting the understanding of the reported test results is the lack of physicochemical characterization of the studied particles. In recent years, the understanding of sample preparation issues as well as the significance of nanoparticle physicochemical properties and composition have improved. Chapter 2 of this book contributed by Drs. Jeffrey Clogston and Anil Patri provides a comprehensive overview of critical points relating to nanomaterial characterization prior to their use in immunological tests. Other common issues which may confound the results of immunotoxicological studies are nanoparticle sterility, sterilization, and contamination with endotoxin and synthesis by-products. All these challenges and practical solutions to overcome them are reviewed in detail in Chapter 3 by Dr. Nanda Subbarao, Chapter 4 by us, and Chapter 5 by Dr. David Grainger et al .
2. Interaction with Blood Components
If a nanoformulation is administered via i.v. or distributed into the systemic circulation after administration through other routes, then understanding its interaction with blood components becomes an essential initial step in preclinical development. Blood is composed of plasma and cells, where the plasma constitutes about 50% of blood fluid and is composed of water, mineral ions, dissolved gases (oxygen and carbon dioxide), glucose, and proteins. There are about 3,000 proteins in the plasma.12 When nanoparticles enter the bloodstream, they bind proteins almost instantaneously.13 The protein layer on the particle surface creates a so-called “corona,” which determines particle biodistribution and clear-ance.13 Charged nanoparticles bind more proteins than particles with neutral surfaces. Surface neutralization by means of coating with hydro-philic polymers such as PEG is one of the best studied approaches to protect the nanoparticle surface from protein binding and extend their circulation in the bloodstream. The role of nanoparticle physicochemical properties such as size, charge, hydrophobicity, composition, dynamics, and the significance of opsonization in nanoparticle biodistribution and clearance are discussed by Drs. Lennart Treuel and G. Nienhaus in Chapter 6.
The cells in blood include erythrocytes, monocytes, granulocytes, lymphocytes, and thrombocytes (also known as platelets). Hemolysis (damage of red blood cells), coagulation (blood clotting through effects on coagulation cascade, platelets, endothelial cells, and leukocytes), and activation of the complement system are commonly used parameters to test nanoparticle hematotoxicity.
2.1. Hemolysis
This term refers to the potential of a nanoparticle to damage erythrocytes. The loss of red blood cells following hemolysis may lead to anemia, and the release of iron-containing protein hemoglobin into the circulation may lead to nephrotoxicity.14 This is why optimizing the nanoparticle formulation to prevent hemolysis is essential to avoid such complications. Nanoparticle interactions with red blood cells are largely determined by their physicochemical properties, which may differ among different classes of engineered nanoma-terials. For example, the hemolytic activity of silver colloids with an identical surface charge was shown to depend on particle surface area, in that particles with a smaller size and larger surface-to-volume ratio were more toxic in the hemolysis assay than their larger counterparts.15 The mechanism of toxicity of anionic silver nanoparticles was attributed to the greater release of silver ions from particles with a greater surface area.15 Hemolysis caused by silica nano-particles was recently attributed to surface charge, with cationic species being more damaging, as well as to porosity and shape in that mesoporous particles with a large aspect ratio were less hemolytic than spherical particles and mesoporous particles with a low...