
- 360 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About This Book
The book highlights recent prominent results in the domain of the synthesis of new polyoxometalates with a specific attention to polyoxothioanions, and provides some novelties and perspectives in selected domains such as magnetism, luminescence and nanochemistry, and macroions self-assembly in solutions. The case of “one-pot” syntheses often used and reported in POMs synthesis is studied in terms of more complex solution speciation processes related to highly dynamical situation connected to factors such as pH, ionic strength, reaction time, temperature, counterion nature, concentration of starting materials, presence of electron donors and redox potentials. The behavior of macroions (2nm-6nm size range) in solution is shown to be quite different from the simple ionic solution or colloidal systems (Debye–Huckel model). Their self-assembling into a single-layered, spherical, hollow vesicle structure, namely the “blackberry” structure, is clearly described. Examples of spin clusters with tunable interactions are given and single molecule magnets based on POMs are specifically tackled. Besides paramagnetic transition metal centres and lanthanoid ions encapsulated in archetypal lacunary polyoxoanions, magnetically functionalized Kleperates are described, their discovery tracing back nearly 15 years.
Contents:
- Polyoxometalate-Protected Metal Nanoparticles: Synthesis, Structure and Catalysis (Yifeng Wang and Ira A Weinstock)
- When Giants Meet Dwarves in the Same Pond — Unique Solution Physical Chemistry Opportunities Offered by Polyoxometalate Macroions (Dong Li, Panchao Yin and Tianbo Liu)
- Directed Assembly of Polyoxometalates Across Length Scales: From Macro-Molecules to Microsystems and iChells (Antoine G Boulay, Geoffrey J T Cooper and Leroy Cronin)
- Magnetic Polyoxometalates (Juan M Clemente-Juan, Eugenio Coronado and Alejandro Gaita-Ariño)
- Magnetism of Keplerates (Paul Kögerler)
- Polyoxometalates as Ligands for Functional Lanthanoid Complexes (Chris Ritchie and Colette Boskovic)
- Polyoxothiometalates POTM (Francis Sécheresse and Emmanuel Cadot)
Readership: Graduate students and researchers of nanoscience and nanotechnology and chemistry (physical and inorganic) — inter-disciplinary.
Frequently asked questions
Information
Jinan 250199, PR China
POB 653, Beer Sheva, 84105 Israel
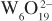
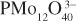
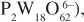
Table of contents
- Front Cover
- Half Title
- Series Title
- Title Page
- Copyright
- Preface
- Contents
- List of Color Plates
- Chapter 1 Polyoxometalate-Protected Metal Nanoparticles: Synthesis, Structure and Catalysis Yifeng Wang and Ira A. Weinstock
- Chapter 2 When Giants Meet Dwarves in the Same Pond — Unique Solution Physical Chemistry Opportunities Offered by Polyoxometalate Macroions Dong Li, Panchao Yin and Tianbo Liu
- Chapter 3 Directed Assembly of Polyoxometalates Across Length Scales: From Macro-Molecules to Microsystems and iChells Antoine G. Boulay, Geoffrey J. T. Cooper and Leroy Cronin
- Chapter 4 Magnetic Polyoxometalates Juan M. Clemente-Juan, Eugenio Coronado and Alejandro Gaita-Ariño
- Chapter 5 Magnetism of Keplerates Paul Kogerler
- Chapter 6 Polyoxometalates as Ligands for Functional Lanthanoid Complexes Chris Ritchie and Colette Boskovic
- Chapter 7 Polyoxothiometalates POTM Francis Sécheresse and Emmanuel Cadot
- Index