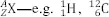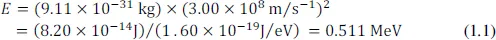![]()
Chapter 1
Introductory Atomic and Nuclear
Physics
1.1 Introduction
We have become so accustomed to the idea that matter is made of tiny parts—molecules, atoms, and electrons—that it is somewhat of a surprise to realize that this fact became accepted just over a hundred years ago. Indeed it is rumoured that Ludwig Boltzmann, depressed that the rest of the scientific world did not share his belief in the reality of molecules, committed suicide in 1906. Boltzmann had shown that the results of classical thermodynamics could be beautifully explained by the assumption that matter consists of small, indivisible, weakly interacting molecules. The equation that appears on his gravestone —
S =
k ln
W — summarizes his work; it relates the macroscopic entropy (
S) of a system to the number of microscopic states (
W) that the system can access, where
k is Boltzmann’s constant.
Of course, Boltzmann acted precipitously, since there were many supporters of the theory that matter consisted of indivisible atoms or molecules: Democritus in Greek times, Newton in the 17th century, Dalton and Avogadro in the 19th. Although some scientists continued to deny reality to objects that could not be observed directly, experimental results were against them; atoms and molecules are much more than merely mathematical fictions. Even more astonishingly, experiments showed that the atom was not indivisible; atoms, apparently, consist of other, even smaller objects, whose reality is also incontrovertible.
1.2 The discovery of the nucleus
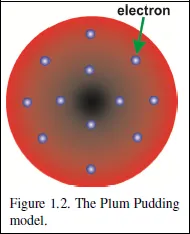
In 1897, J.J. Thomson of Cambridge University was studying the passage of electric current through dilute gases contained in glass tubes called discharge tubes. He noticed that particles were emitted from the negative poles—the cathodes—of the tubes. These particles were shown to have a negative charge; we now call them electrons. Since they are emitted from atoms, it seems probable that they are constituents of the atom. Since the atom is neutral, the rest of the atom has to have a positive charge. Accordingly, Thomson proposed the ‘Plum Pudding’ model, in which the electrons are embedded in a uniform sphere of positive charge, like the plums in a pudding. That this model was a poor one was demonstrated by the brilliant experiments of Ernest Rutherford and his collaborators.
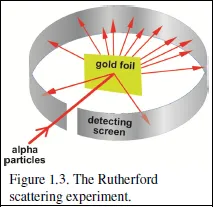
In 1896, the French physicist Antoine Henri Becquerel, noticed that some rocks that he had been studying emitted radiation that could pass through opaque photographic paper. Ernest Rutherford (“the father of Nuclear Physics”), a New Zealander, and one of Thomson’s students, identified two of these radiations. He showed that the first, which he called alpha radiation, is nothing but Helium atoms with two electrons removed, with, therefore, a positive charge of plus two. His experiments using these alpha particles showed conclusively that the mass of the atoms is almost totally concentrated in a tiny ‘nucleus’ at the centre.
The experiments were simple in principle: a beam of alpha particles from a radioactive source were directed at a thin gold leaf. If the Plum Pudding model represented reality, the alpha particles would beexpected to suffer only many small deflections as they encounter the spread-out charge of the gold atoms. However, Rutherford, much to his astonishment, noted a significant number of very large deflections, some of them even causing the heavy alpha particles to bounce back from the foil.
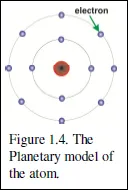
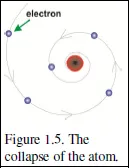
Thus was born the Planetary Model of the atom, in which the electrons circulate around a tiny but very massive nucleus, rather as the planets circulate round the sun.
Unfortunately, Rutherford’s planetary model has an apparently fatal flaw. The accelerating electrons that were supposed to orbit around a central nucleus would, according to classical physics, lose energy by emitting electromagnetic radiation (see
Appendix A1.1); this loss of energy would cause them to spiral inward to the nucleus till the atom collapsed. That we are here to observe our world lets us know that this does not happen—so what’s going on here?
There was another difficulty. Atoms do indeed emit electromagnetic radiation. However, rather than a spectrum that has a continuous range of energies, as classical physics predicts, this radiation appears only at a finite number of discrete energies, shown in Figure 1.6. Both difficulties were ‘solved’ by Niels Bohr, the Danish genius (§1.5).
1.3 The basics of nuclear physics
We now know that the nuclei of atoms consist of
protons with positive charge, equal and opposite to that of the electron, and
neutrons with charge zero; together these are called
nucleons. The number of protons in a nucleus is denoted by
Z and the number of neutrons by
N.
Z is called the
atomic number of the nucleus since it indicates where on the table of the elements that particular one lies. The
mass number of the nucleus—the total number of nucleons—is denoted by
A, which equals
N+Z. Then a particular nucleus
X is written as
etc. Since
Z is entirely determined by the chemical symbol, this is often abbreviated by omitting the
Z value, as in
12C,
16O: pronounced “Carbon–12, Oxygen–16”. Nuclei that have the same value of
A are called
isobars.
The value of Z determines the chemical behaviour of the element. However, different nuclei can have the same value of Z, yet different values of N (and thus of A. Nuclei with the same Z but different A are called isotopes. Thus 12C, 11C, and 14C are isotopes of Carbon. Isotopes are either stable or unstable; the unstable ones emit radiation, as we shall see in Chapter 3, and are called radioisotopes.
Rutherford’s experiments indicated the approximate size of the gold nuclei he used as targets. Later experiments showed that the nuclei of all atoms are roughly spherical with a radius that depends on the number of nucleons. This radius is given by R = r0 A 1/3 where r0 ≈ 1.2 x 10−15 m. (10−15 m is defined as one fermi after the famous Italian physicist; it is also one femtometre; the abbreviation is fm). This is an astonishingly small number in comparison to the radius of the atom of about 10−10 m.
Since nuclei hold together, in spite of the Coulomb repulsion between the positively charged protons, it is necessary to postulate another force of nature—the so-called strong force—that comes into play only at a distance of less than 10−15 m or so. This very short-range strong force does not distinguish between neutrons and protons, being attractive between any two nucleons (p–p, p–n, n–n); it is the glue that holds the nucleus together. For very large nuclei, the peripheral protons can lie outside of the range of the strong force and their mutual Coulomb repulsion tends to pull the nucleus apart. However the strong attractive forces between the neutrons tend to moderate this effect. The interplay of these opposing forces sets a limit on the size of stable nuclei.
Just as we would need to apply energy to raise a stone out of a hole in the earth, energy is needed to pull a nucleus apart. Since Albert Einstein showed that, energy is mass (§1.4.2), this means that the sum of the masses of the individual protons and neutrons of which a nucleus is composed is greater than the mass of the nucleus. The difference between the sum of the masses of the nucleons and the mass of the nucleus that they make up, expressed as energy, is called the binding energy of the nucleus.
1.4 Notes on units
1.4.1 The electron volt
The SI unit of energy, the joule, is too large a unit to be useful in atomic or nuclear physics. Accordingly we define the electron volt (eV), the energy that an electron would acquire when accelerated through a potential difference of one volt. The electron is now known to have a charge of e = 1.60 x 10−19 Coulombs (first measured by Robert Andrews Millikan’s famous oil-drop experiment in 1909). Thus the electron volt is related to the joule by 1 eV = 1.60 x 10−19 joules.
1.4.2 The most famous equation in the world
For the very small masses encountered in atomic or nuclear physics, the kilogram is not a useful unit (the mass of the electron, for example, is 9.11 × 10−31kg). A more useful unit is defined using the most famous equation in physics, E = mc2, a result of Einstein’s theory of Special Relativity (1905). E is the energy, m is the mass, and c ≅ 3 × 108m.s−1 is the velocity of light. This equation quantifies the fact that mass and energy are equivalent, so that mass can be expressed in energy terms. For example, if we could somehow completely annihilate an electron, Einstein’s equation shows that the energy would be:
Thus we can write the electron mass (me = E/c2) as 0.511 MeV/c2. MeV/c2 is a useful unit, since it gives at a glance how much energy a particular mass could yield.
1.4.3 The a...