![]()
Chapter 1
Physiology of Stem Cells in the Hippocampal Dentate Gyrus
J. Martin Wojtowicz and Yao Fang Tan
Introduction
Physiology of stem cells does not exist. This was our first thought when asked to write an introduction to this volume. A prevalent dogma is that stem cells are quiescent, “mysterious” cells endowed with the phenomenal powers of multipotentiality and self-renewal. These cells give rise to progeny when commanded by neurotrophins and growth factors, but are otherwise unique, independent, and not really a part of traditional hippocampal system. However, gradually, as the writing progressed we began to realize that indeed there is a physiology of stem cells and it amounts to the same physiology that is described in neuroscience textbooks dealing with brain in general, and hippocampus in particular. Although stem cells do not produce action potentials and do not express neuronal proteins, they are very much a part of the brain circuit. This participation is somewhat analogous to glial cells that were once thought to be passive and separate, but are now recognized as full-fledged partners in the brain circuitry working in tandem with neurons. In fact, stem cells express glial fibrillary acidic protein (GFAP) previously thought to be a sole characteristic of astroglia. Moreover, despite a lack of synapses, stem cells express typically neuronal receptors for the major central inhibitory transmitter gamma aminobutyric acid (GABA), and react to activation of these receptors by opening chloride channels and subsequent depolarization. This latter characteristic is similar to that of young neurons which are in large measure controlled by GABA as they progress through developmental stages of proliferation, migration and differentiation (Kilb et al., 2013).
The evidence summarized in this chapter will show that stem cell physiology is inseparable from the physiology of the rest of the dentate gyrus (DG) and consequently the rest of the hippocampus. In addition to being the source of new neurons, stem cells are far more responsive to the activity of the surrounding neuronal circuitry than previously thought. At the membrane level, downstream responses to membrane depolarization and hyperpolarization lead to fundamental changes in the cell’s behavior. Membrane depolarization from the resting state stimulates asymmetric division of stem cells to effectively produce neuronal precursors in the form of neuroblasts, which can divide further and ultimately develop into mature neurons. Membrane hyperpolarization results in an increased rate of symmetric divisions by mitosis and consequently increased replication of stem cells. Such responses of stem cells to changes in membrane potential represent a form of brain plasticity because the production of neuroblasts can have profound physiological consequences and elicit behavioural outcomes. Further, the physiological significance of stem cells is emphasized by their presence in the hippocampal DG, which is a gateway to the hippocampus, the key brain structure involved in learning and memory.
Essential Steps in Neuronal Development
Stem cells possess two basic properties, self-renewal and multipotentiality. Within the adult brain, there are two main regions where stem cells take residence during late embryonic development and persist postnatally for as long as the animal lives. The region that produces most progeny is the subventricular zone (SVZ) of the lateral ventricles. It has been estimated that as many as 50,000 cells are produced daily in the SVZ of adult laboratory rodents. Many of these cells die shortly after the first division but the remaining tens of thousands are carried via the rostral migratory stream (RMS) to the olfactory bulbs where they develop into functional interneurons (Lledo, 2011; Platel and Bordey, 2011) An analogous process occurs on a smaller scale in the hippocampus, a brain region that controls many forms of learning and memory. The migration of neuronal progenitors from the subgranular zone (SGZ) within the DG is limited to the granule cell layer, which measures only 100 μm in width so the migration path is relatively short. Nevertheless, the control mechanisms affect progenitor migration, proliferation, differentiation, maturation and survival. Collectively, these steps are known as adult neurogenesis (ANG). The name would suggest the process is very different from embryonic neurogenesis, but this is far from certain. In fact, many factors are known to control both embryonic and adult neurogenesis in a similar way. It is not even certain when one developmental process really ends and the other begins because in many species, including laboratory rodents such as mice and rats, the embryonic development of DG carries into a postnatal phase lasting prominently for 1–2 weeks after birth.
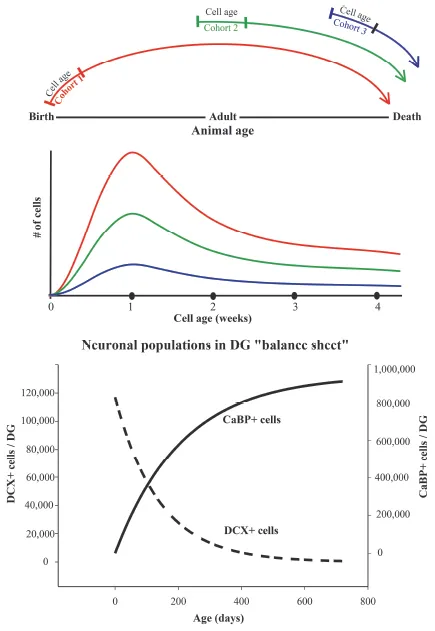
This period may be different in other species where the brain develops primarily in embryo, but it usually carries into the postnatal period to some extent (Kempermann, 2012). There is a phase in the animal’s development when embryonic, postnatal and adult neurogenesis (ANG) appear to overlap. However, ANG proper presumably takes over when the animal reaches sexual maturity. This initial overlap may cause confusion when studying factors controlling cell development and those primarily controlling the animal’s development. It would appear that ANG can serve different functions in animals of different ages, as would be expected, but superimposed on this age-dependence is the flexibility of the ANG process itself. The four crucial steps in each cell’s development, which include proliferation, differentiation, maturation and survival, occur at different rates and are controlled uniquely according to the animal’s age. Hence, it is crucial to always present and discuss ANG in terms of these steps and also as it relates to the animal’s age. To emphasize this distinction, cell development should be viewed on a sliding scale of animal’s development and its inevitable aging (see Figure 1).
Figure 1. Sliding scale for adult neurogenesis (ANG). Top: Physiology and regulation of ANG is related to both the animal’s age, and the cell age of each successive cohort. The early postnatal cohort is developing among numerous surrounding neurons of approximately the same age and in the context of high demand for hippocampal learning as the animal experiences the environment for the first time. The adult and aging cohorts experience a different environment, where the neuronal circuitry is mostly fully developed with very little free space so the new neurons have to displace the pre-existing cells. Because new neurons are a small minority among the mature ones, the microenvironment, essentially a support system in the sub-granular zone, may not be the same as in the young animal. The surrounding neuronal circuitry within the dentate gyrus (DG) also changes with age. The amount of GABA-ergic inhibition, and the synaptic density of excitatory inputs are reduced as the animal ages. Physiological/behavioural demands for new neurons in the mature animal may also be very different. Thus, the amount of new learning in the adult may be less than in a young animal, but recall of the pre-existing memories may be in high demand. Hence, the involvement of adult-born neurons in learning and memory processes may be animal and age specific. Middle:Survival curve for new neurons. For each new cohort of neurons shown in the top panel, the cell number is maximal during the first week, due to proliferation of neuroblasts. During the second week, cell numbers decline due to apoptosis. Cells that survive this decline persist for the rest of animal’s life. The shape of the survival curve is similar for cohorts born in young (red), middle aged (green) and old (blue) animals but the overall number of produced cells is much smaller for the latter. Bottom:Balance sheet for ANG. The graph shows declining number of young neurons expressing doublecortin (DCX+) based on cell counts in rats of different ages. The rising curve represents the calculated number of neurons at 4 weeks of cell development. These neurons express calbindin (CaBP+), a mature neuronal marker Data is based on quantitative measurements of cells numbers in (McDonald and Wojtowicz, 2005). Note that in spite of reduced number of immature neurons, the addition of new neurons is still significant in adult animals due to a delay between cell birth and cell maturation.
Activity-dependent Regulation of ANG
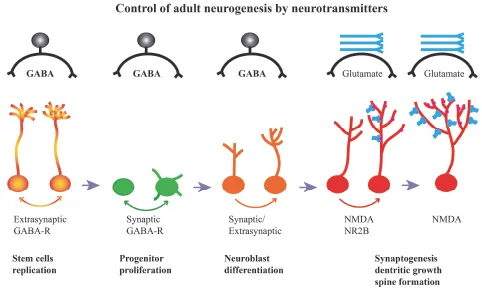
Physiological/environmental regulation of ANG begins with stem cells (Figure 2). This is potentially the most powerful mode of regulation since in addition to changes in the rate of cell division, the cell fate can also be altered. Social isolation has been shown to increase the proportion of stem cells to neurons within adult hippocampus (Dranovsky et al., 2011) whereas enriched environment had an opposite effect (Song et al., 2013). The neurotransmitter GABA is a powerful regulatory factor at this early stage of cell development, and is in plentiful supply since it is a major inhibitory neurotransmitter utilized by hippocampal interneurons. Conveniently, there is a wide range of interneuron types with different soma locations, axonal arborizations and membrane properties (Freund and Buzsaki, 1996). It is well known that these interneurons can affect the activity of mature granule neurons but it is also becoming apparent that at least some of these interneurons affect the stem cells as well as adult-born, immature granule neurons (Armstrong et al., 2011; Markwardt et al., 2011). A subtype of these interneurons, basket cells, expressing a co-transmitter parvalbumin (PVA), are known to make synaptic contacts with proliferating cells in the SGZ (Song et al., 2013). Stimulation or inhibition of these PVA interneurons can alter the rates of neuroblast proliferation and their differentiation into young neurons. Independently, stem cell division is altered by GABA release from PVA interneurons, but in this case the effect is mediated by extrasynaptic GABA receptors containing gamma-2 subunits (Song et al., 2012). Activation of these receptors by ambient GABA promotes asymmetric division of stem cells. The resulting increase in the number of neuronal precursors can in turn lead to a subsequent increase in the number of new neurons. In contrast, inhibition of gamma-2 subunit-containing receptors or reduced concentration of GABA in their vicinity can promote symmetric stem cell replication at the expense of neuroblast production, but this only occurs in a specific neurogenic niche within the lower blade of the dentate gyrus (Dranovsky et al., 2011).
Figure 2. Regulation of ANG by GABA and Glutamate. Progression from stem cells to fully functional neurons is regulated by neurotransmitter GABA during early stages of cell development. This regulation is stage-specific and mediated by either synaptic or extrasynaptic GABA receptors. In some cases, a particular type of participating interneurons and subtype of GABA receptors has been identified (see text). At later stages of development glutamate, acting on glutamate (NMDA) receptors, regulates the rate of synaptogenesis, synaptic strength, dendritic growth and spine formation.
The influence of GABA on ANG continues at later stages of cell development. A synthetic GABA agonist 4,5,6,7-Tetrahydroisoxazol [5,4,-c] pyridine-3-ol hydrochloride (THIP), also known as Gaboxadol, with its preferential affinity for delta GABA receptors stimulates neuronal production when given to the animal during the second week of cell development. At this stage, neuroblasts have stopped proliferating, express young neuronal marker doublecortin (DCX) and undergo a critical period for cell survival and differentiation (Whissell et al., 2013). In support of this mechanism, the delta GABA receptor knockout mice show reduced neuronal maturation, and behavioral phenotype consistent with deficient ANG.
Glutamate takes over control of cell survival and growth when GABA influence ends. During the period between 3–6 weeks of cell development, new neurons are under control of NR2B-subtype of N-methyl-D-aspartate (NMDA) glutamate receptors. At later stages (two months and more) of cell development, glutamate-dependent neuronal plasticity persists, but at that time the main forms of plasticity being controlled by glutamate are synaptogenesis and dendritic growth (Tronel et al., 2010).
Behavioral experiments provide supporting evidence for these regulatory mechanisms by GABA and glutamate. As noted above, social isolation and environmental enrichment have opposite effects on the stem cell population (Dranovsky et al., 2011), consistent with the regulatory mechanism proposed by Song et al. 2012. At this stage, GABA is thought to be released into the extracellular space and act on extrasynaptic gamma-2 receptors. In addition, enriched environment has an effect on neuroblast expansion and ultimately on neurogenesis, presumably by activation of synaptic GABA receptors via PVA interneurons (Song et al., 2013). Similarly, exposure to an enriched environment stimulates the formation of excitatory synapses onto the developing neurons. This process involves, at least partly, recruitment of previously silent NMDA synapses to more active status and consequent endowment of synapses with functional a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (Chancey et al., 2013). At later stages of neuronal development, adult-born neurons are primarily under the influence of excitatory, glutamatergic synapses and this regulation can involve rather specific environmental stimuli. In case of spatial learning for example, learning stimulates survival of relatively well-developed new neurons while inhibiting a less mature cohort (Dupret et al., 2007). In addition, proliferation of neuroblasts at an even earlier stage of development is stimulated (Dupret et al., 2007). Thus, control of neurogenesis by brain activity can have a complex pattern and is stage-sensitive.
Not surprisingly, in view of a tight relationship between newly born neurons and the existing circuitry of the DG, learning and memory can be influenced by changes in ANG. Upregulation of ANG by THIP, acting via GABA-delta receptors, facilitates the learning of contextual discrimination (Whissell et al., 2013). In contrast, reduced ANG during aging or in pathological cases (i.e. Alzheimer’s disease) results in impairment of memory (Drapeau and Abrous, 2008; Winocur et al., 2014).
Specificity of regulatory mechanisms is partly dictated by the topography of the circuitry within the DG. Considering that GABA mediates regulation of the early stages of neurogenesis, it becomes apparent that such regulation may be relatively widespread along the longitudinal and transverse axes of the hippocampus due to wide topographical ramifications of the axonal arbour of the inhibitory interneurons (Figure 3). These arbours are often limited to certain regions of the DG as viewed in transverse hippocampal sections, but nevertheless cover large proportions of the DG and presumably make contacts with t...