![]()
CHAPTER 1
Gene Therapy
Definition
Gene therapy is the treatment of disease by repairing or reconstructing defective genetic material. This technique was originally designed to correct human genetic disorders caused by abnormalities in the genetic material. Such disorders can be caused by abnormalities in a single gene (for the definition of “gene,” see below), a combination of abnormalities in several genes, abnormalities in the chromosome structure, or mutations in the genetic material of mitochondria (cell organelles that produce energy). Current gene therapy technology focuses primarily on the correction of abnormalities caused by a single gene or by several genes. Therefore, modern gene therapy can be defined as a technique that replaces one (defective or undesirable) gene with another (normal or therapeutic) one. It is important to note that this technology is not intended to change the human genome (the collection of all genes), nor to introduce any non-human genes into human cells. This is what distinguishes gene therapy from genetic engineering, which is intended not just to repair an existing genome, but to create new genomes that do not exist in nature. Gene therapy has now been under development for several decades, but so far it has not produced any clear-cut therapeutic results, and it can still be considered to be in its infancy.
Each year, over 150,000 babies born in the US, and an estimated, 3,000,000 babies worldwide, have birth defects. More than 6,000 single-gene disorders are currently known. Among the disorders are cystic fibrosis, sickle cell anemia, Huntington’s disease, hemophilia, and others. Each and every person carries at least several defective genes. Fortunately, most of these defects are not life-threatening, and do not cause any obvious abnormalities. However, this has a down side: Defective genes that do not cause serious defects or death are easily transmitted from parents to children. Also, as humans learn how to treat genetic disorders, more and more people carrying defective genes survive and pass them through successive generations, leading to the gradual accumulation of gene defects in future generations. For example, the genetic defect that causes hemophilia: People with hemophilia lack a particular protein involved in blood clotting, and thus lack the ability to stop bleeding. Even minor injuries that are harmless to most people can be fatal to hemophiliacs. However, if hemophiliac patients receive appropriate care, they will survive and pass on the defective gene. A good illustration of this comes from the European royal families, in which the defective gene was easily passed from Queen Victoria to the royal families of Russia, Prussia, and Spain.
The human genome consists of 23 pairs of chromosomes (46 chromosomes total). One of the chromosomes from each pair originates from the mother, and the other one from the father. Thus, each gene is present in duplicate, or pairs (one copy from each parent). Very often however, one gene from a pair “contradicts” the instructions of the other. For example, one gene might instruct the eyes to be blue, while the other gene carries the instructions for brown eyes. In such situations, the body has to decide which gene controls eye color. The controlling gene is called the “dominant” gene, while the second (silent) one is called the “recessive” gene. In this particular case, the gene responsible for brown eyes is dominant, while the gene responsible for blue eyes is recessive. In some situations, a defect in a single dominant gene will cause a problem. On the other hand, if a single recessive gene is defective, it will be compensated for, if it is paired with a normally-functioning dominant gene, therefore preventing harm. However, if an individual is unfortunate enough to inherit a defective recessive gene from each parent, resulting in two recessive genes, then a disease can arise because there is no normal dominant gene to compensate the defective recessive gene.
The pair of sex chromosomes is an exception. Females have two X chromosomes (XX), while males have one X chromosome and one Y chromosome (XY). Thus, genes in the male pair of chromosomes are not duplicated, and defects in the X chromosome cannot be compensated by the Y chromosome, thereby causing so-called X-linked genetic diseases. Perhaps the best known example of such diseases is hemophilia. If a male inherits the defective X gene, he will always develop this disease, whereas both X genes must be defective to cause hemophilia in a female.
History
Gene therapy for human genetic diseases was first conceptualized in 1972.1 Attempts to correct genes were soon made, but were not successful. An important step in the development of gene therapy technology is the delivery of therapeutic genes into human cells. Viruses can be easily used to transport therapeutic genes but, unfortunately, they normally cause diseases. Fortunately, a breakthrough came in 1984, when viruses were altered in such a way that they became safe for use in human gene therapy procedures.
The first gene therapy clinical trial was performed in 1990 in the US, when a patient was treated for ADA-SCID (for more, see Ref. 2). Over 1,000 other clinical trials were approved worldwide since then, most of them in the US.3 Unfortunately, none of them have been considered completely successful to date. Even worse, several trials had to be stopped due to the danger of side effects, such as cancer and death. The first blow to this research came in 1999, when an 18-year old man died in the US in a trial targeting ornithine transcarbamylase deficiency (OTCD). He died from multiple organ failure just four days after the treatment, and it is believed that his death was triggered by a severe immune response to the adenoviral vectors (defined below) that were used to deliver the therapeutic gene. Another setback came from France in 2003. In this trial, 11 boys were treated using retroviral vectors for X-linked severe combined immunodeficiency (X-SCID). Two of them subsequently developed a leukemia-like condition, which was a side effect of the retroviral vectors.4 Nevertheless, one can argue that this trial was not a failure, as is widely considered, but rather the first success. It seems that the other nine boys out of the total 11 are just fine, while the two boys that acquired the leukemia-like condition are undergoing treatment and may recover.
These side effects and failures prompted authorities around the world to take a fresh look at gene therapy trials and to put some of them on hold. As a result, the number of approved gene therapy clinical trials declined from 116 in 1999 to just 73 in 2011.3 Although this slowed down progress in western nations, it did not affect some others. In 2003, China’s State Food and Drug Administration approved the first gene therapy product for clinical use in humans.5 This product, Gendicine, is used against various forms of cancer (see more details in Applications). Also, the first such product has been finally approved in the West. The European Commission has recently given authorization to the small Dutch company, uniQure, to treat people with a rare genetic disorder, beginning of summer 2013 (see more details in Applications).
Principles
Gene therapy is done by replacement of a defective gene with a normal one. To deliver the genes into certain types of cells, and into the appropriate place inside of the cells, viruses and other carriers are used. Therefore, to understand how gene therapy works, one must first know what a gene is, and then how it can be transferred using viral and non-viral carriers.
(1) Gene — Genes are stored in DNA, a component of chromosomes, which are located in the nucleus of a cell. Some genes are also located in another cell compartment, called mitochondria. However, the main function of mitochondria is to produce energy for the cell. Also, human mitochondria have only 37 genes, as opposed to the approximately 30,000 genes contained in the nucleus.
DNA contains all of the instructions for cell functions. To “read” these instructions, pieces of DNA are first copied (transcribed); the copies, called RNAs, are then usually transported from the nucleus into the cytoplasm, where they instruct the cell to produce proteins, which serve as the functional building blocks of the body. Traditionally, a piece of DNA that encodes a protein was called a gene. Recently, however, this definition has expanded to include segments of DNA that encode RNAs, because not all RNAs carry the instructions to build proteins. Some RNAs are used to produce proteins and other RNAs serve as the regulators. Currently, the most acceptable definition of a gene is: a DNA sequence that encodes an RNA molecule. However, the discussion over the definition of “gene” will continue to evolve as more is learned.
The collection of all genes from one single cell is called a genome. With few exceptions, genomes of cells originating from the same organism are identical, no matter from which organ or tissue. Let us take, for example, your own body: Genomes of neuronal and muscle (or any other) cells taken from your body will be 100% identical, despite the fact that these cells look completely different. One notable exception is certain cells comprising the immune system, where the DNA rearrangements occur in different patterns in different immune cells. This mechanism allows immune cells to recognize a large variety of foreign substances. On the other hand, the genome from your neuronal cell and the genome from the neuronal cell of any other person (except in case of identical twins) will be slightly different, even if the cells appear identical. The slight differences in the genome from one person to the next are responsible for the unique characteristics of each individual. Nevertheless, all human genomes are at least 99% alike.
How does it happen that nearly all cells in the same body have the same genes, but they look different, and they do different jobs? This is because only certain groups of genes are active in any cell at any given moment, while the rest of the genes are “silent.” As cells develop and grow, different genes become active or inactive. Actually, it would be correct to say that gene activation and inactivation prompt cells to develop, grow, or to do whatever else. The set of active and inactive genes in cells determines both how they look and what they do.
The human genome contains about 30,000 genes. The genome is stored in the cell nucleus, which can be easily seen under a light microscope. Nuclei contain smaller structures, called chromosomes. Chromosomes are composed of DNA as well as proteins, and proteins have a supportive function in chromosomes. In turn, the DNA is composed of four chemicals that are called nucleotides (A, T, G, and C), much like a four-letter alphabet. These four chemicals bind to each other in a specific order to form the sequence of the DNA, for example …ATTTTCCG… and so on. Each DNA strand consists of more than one billion nucleotides, strung together in a precise linear sequence. Amazingly, the linear order of just these four chemicals encodes the blueprint of our life — it determines the color of our eyes, it controls how tall we can grow, and what diseases we are prone to.
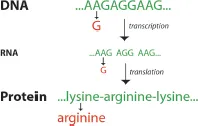
(A) How genes work: A gene is a piece of DNA that tells cells how to do a particular job. Genes instruct cells on how to build RNAs and proteins, and when to start or stop such production. Proteins are not manufactured in the nucleus, where genes are located, but rather in the cytoplasm. Genes send instructions from the nucleus to the cytoplasm using molecules called RNAs (see Fig. 1.1). Pieces of DNA, called genes, are copied in the nucleus (this process is called transcription). The copies, called messenger RNAs, are then transported into the cytoplasm, where they are used as instructions to build proteins (this process is called translation). Proteins are built from chemicals called amino acids. The nucleotide sequence of an RNA determines the amino acid sequence of the corresponding protein. Specifically, each consecutive group of three nucleotides of RNA provides the code for an amino acid that will be used next to build a protein. For example, the AAG nucleotide sequence instructs that the amino acid lysine should be used to build a protein, while the AGG sequence will instruct that the amino acid arginine should be used.
Fig. 1.1Genes instruct the synthesis of proteins. In this particular example, the gene sequence …AAGAGGAAG… instructs that the sequence of a synthesized protein should be lysine-arginine-lysine. Any single mistake in the gene sequence can lead to the synthesis of a protein that has the wrong sequence. For example, the exchange of the second nucleotide A for G will lead to the synthesis of a protein in which the amino acid lysine is replaced by the amino acid arginine, which consequently might cause a protein to function abnormally or not to function at all.
The vast majority of DNA (about 97%) contained in human chromosomes does not make up genes. The role of this non-genic DNA is not yet understood. This DNA might regulate the expression of genes. It might also represent old ancestor genes, as well as new evolving genes, and it might be necessary for gene evolution. Most research is currently devoted to genes only, without serious consideration for the remaining more than 90% of DNA. Step by step, genes are sequenced, and we learn how they work. But even if one discovers them all, science might still be far from understanding exactly how the human genome works, because we know almost nothing about the rest of the DNA.
(B) How to make genes: In order to synthesize a gene, we need to know its DNA sequence. In other words, the gene must first be decoded. Based on the sequence, we can make genes in several ways. The first step, however, is always the same — DNA that contains genes must be isolated from cells, and then purified. Then, DNA fragments representing the gene of interest either can be “cut out” of the total DNA using special enzymes, or they can be synthesized by a technique called “polymerase chain reaction” (PCR).
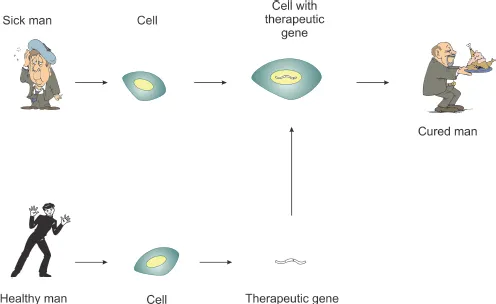
(C) Where to deliver : Depending on the goals of the therapy, genes can be transferred either into germ (egg or sperm) cells or into somatic cells (any other cells in our bodies). When genes are transferred into someone’s germ cells, they can be passed on to the offspring, while genes transferred into somatic cells cannot be passed on. The germ-line gene transfer is widely used for manipulations with animals, but not with humans, because of technical and ethical issues. Most of the current human gene therapy trials are performed using somatic cells, as shown in Fig. 1.2.
(D) How to deliver: Delivery of therapeutic genes into cells is not an easy task. Cells are covered with membranes that isolate them from the environment, and allow only select molecules to penetrate. Membranes do not let manufactured or foreign DNA fragments (representing laboratory isolated genes) to penetrate cells. Therefore, genes must be forcefully delivered across membranes, and several methods currently exist to do this. One way is to isolate a fragment of DNA containing the desirable genes, then break the membrane, and allow the genes to freely flow inside the cells, hoping that they will eventually integrate into the host DNA and repair the defective genome. Another method involves special delivery molecules called “vectors.” In this case, DNA with a desirable gene is first inserted into the vector, and then the vector delivers the gene into cells. Currently, the most popular vectors are viruses. However, viruses can transfer only relatively small DNA fragments, and scientists are working to develop artificial chromosomes that can be used as vectors, in order to operate with larger DNA fragments.
Fig. 1.2 Principles of somatic cell gene therapy. To correct a defective gene, cells are isolated from a sick patient who has the defective gene, and the normally functioning gene isolated from a healthy man is then delivered into these cells. The cells with the new normal gene are then transplanted back into the sick patient, where they divide and replace cells with the defective gene, curing the patient.
(2) Viral Gene Delivery — DNA or RNA, which contains genetic information, is the main component of viruses. Viruses “know” how to exploit human cells. They penetrate into human cells, integrate their genetic information into the host genome, and use the cells to reproduce themselves. Humans are now learning how to exploit viruses. Scientists can now isolate therapeutic human genes and insert them into the viral genome while taking the toxic viral genes out of the viral genome. These “disarmed” viruses t...