![]()
Section 1
Macroscale
![]()
Chapter 1
Enhanced Cryoablative Methodologies
John M. Baust, Anthony T. Robilitto, Andrew A. Gage and John G. Baust
1. Introduction
Cryoablation (cryosurgery) (CA) is an energy-deprivation therapy that relies on the application of a heat-sink to targeted tissues with the expectation of freeze destruction. The origin of modern CA is commonly attributed to the development of a closed-end cryoprobe in which liquid nitrogen (LN2) was circulated while placed within a tissue target [Cooper and Lee, 1961]. Cooper taught that 1–2 minute tissue exposure to −20℃ was adequate to form a necrotic lesion in the brains of patients suffering from motion disorders (i.e. Parkinson disease). Future studies identified the variability of tissue responses to freezing and extended the lethal temperature to a <−40℃ nadir [see review: Gage and Baust, 1998]. More recently, Baust et al. [2014a] reviewed the diversity of CA mechanisms critical to clinical efficacy. Three methodological approaches with an emphasis on cancer ablation are discussed in this chapter. First, elucidation of the mechanisms of cancer cell death following a CA insult will be expanded beyond the initial understanding of necrosis as the path of cell death. Second, the engineering advancements in cryosurgical device technologies are reviewed. These include subcooled LN2, supercritical nitrogen and Joule–Thompson cooling. Third, the use of cryosensitizers, when applied in concert with freezing to optimize a tissues ablative response, is discussed.
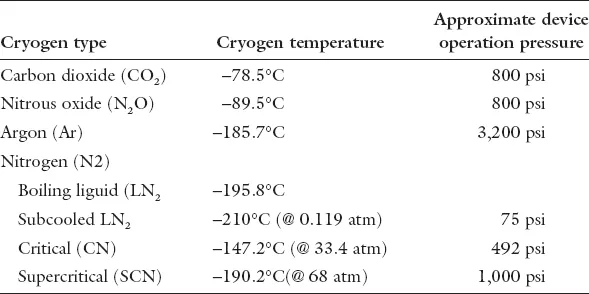
On the surface CA appears to be a rather simple method to ablate unwanted tissue. With an appropriate cryogen (Table 1) tissue temperature is “targeted” to a pre-determined nadir in an effort to assure destructive ice formation. In practice, cells of diverse tissues demonstrate different susceptibilities to freezing [Gage and Baust, 1998] with lethality ranging between −2℃ to −70℃ and further influenced by cooling rate, hold time at nadir temperature, rates of thaw and tumor cell biology. With the exceptions of radical surgery and CA, all other therapies rely on “energy additive” strategies (i.e. radiation, radio frequency, photo dynamic, laser and diverse chemo therapies), which if heat-based, often extend the ablative consequences for up to one week post-treatment [Chu and Dupuy, 2014]. When applied as monotherapies with curative intent, these treatments often yield the suppression of cancer growth for varying periods related to the biology of the disease (i.e. stage of cell cycle, focal vs. extent of dispersion and cancer cell-based defensive responses).
Table 1. Overview of common cryogens used in cryosurgery.
A cancer’s continuous mutation and recruitment of stromal and immune cells to support the growth of a tumor microenvironment presents a “moving target” to treatment which commonly defeats clinically accepted “gold standard” monotherapies [Ghisolfi et al., 2012]. Cancer’s defensive strategies represent evolved capabilities characterized as well-established protective adaptations to anti-tumor efforts [Dimond, 2013; Shackleton et al., 2009]. To successfully manage cancer the activation of multiple cell-type-specific “kill switches” is necessary. Without “kill switch” activation the combined defensive capabilities of the tumor microenvironment provide a strategy to defeat the curative intent of monotherapies [Ghisolfi et al. 2012; Sun et al. 2012]. As an example, radiation therapy for prostate cancer yields >25% positive biopsies in up to one-third of patients requiring secondary radiation treatment following cancer recurrence. A common outcome is radioresistant prostate cancer, the fourth most common genitourinary malignancy [Jone, 2011].
The biology of cancer supports resistance to both radiation and chemotherapy [Sun et al., 2012]. Both therapeutic strategies primarily attack only actively dividing cancer cells in specific stages of the cell division cycle. Non-dividing cancer cells are weakly affected. Further, select tumor cell populations, such as cancer stem cells, have several molecular adaptations in which this sub-population can actively enter or halt the cell cycle, secrete protective proteins, induce extended dormancy, etc. to avoid targeting. Treatment plans must then be repetitive with an aim of inducing cell death in each of the tumor’s sub-populations including dividing, non-dividing, cancer stem cells, dormant cells, etc. [Baust et al., 2015; Moltzahn and Thalmann, 2013]. An unintended consequence of repetitive bouts of treatment is the acceleration of protective (defensive) mutations which progressively result in a treatment-resistant tumor cell population. In the case of cancer stem cells, these protective adaptations can be amplified due to increased molecular plasticity of this population [Dimond, 2013; Shackleton et al., 2009; Sun et al., 2012]. These cells become refractory to secondary re-treatment or alternative attempts at therapeutic intervention [Corn, 2012].
Hence, the development of new therapeutic approaches requires a therapy that is (a) non-repetitive to deny the cells of the tumor microenvironment the ability to activate defensive mutations; (b) capable of simultaneous activation of multiple cell death cascades to limit cancer’s ability to activate survival mechanisms; (c) cell cycle independent; (d) damaging to tumor vasculature and (e) uniform in the destruction of all cells within the tumor microenvironment to eliminate both cancer cell/cancer stem cell survival through dormancy mechanisms. This also allows cryoablation to be a repeatable and even salvage therapy. A critical consequence of cryoablation is the disruption of most of the hallmarks of cancer within tumor cells [Hanahan and Weinberg, 2011] resulting in complete cell death, provided a cancer-specific nadir temperature is reached throughout the tumor microenvironment [Baust et al., 2014a, 2015].
2. Mechanisms of Cell Death
From an historical perspective, cell death following cryosurgery was attributed to two mechanisms, freeze rupture which led to vascular stasis and tissue necrosis, together referred to as “coagulative necrosis” [Bischof et al., 1992, 1993; Cooper, 1965; Gage, 1978; Gage and Baust 1998, 2002; Ikekawa et al., 1985; Smith et al., 1999]. Missing from this concept of “physical damage-related lethality” was recognition of cellular stress mechanisms that may be launched to either support cell recovery or to initiate multiple cell death cascades when repair was not possible [Baust and Gage, 2005; Gage and Baust, 1998; Hoffmann and Bischof, 2002]. Hollister et al. [1998] first observed late stage apoptosis in prostate cancer cells following exposure to elevated sub-freezing temperatures equivalent to those measured in the freeze zone periphery. More recently, Robilotto et al. [2013] demonstrated that not only was necrosis observed throughout the ablative freeze zone but also apoptotic activation was differentiated between membrane- and mitochondrial-mediated cell death pathways based on the intensity of the freeze. While the contribution and importance of the molecular response of cancer cells to the freeze process is now recognized to play a critical impact on outcome, the mechanisms of action are still fairly unknown. This due in part to the lack of detailed molecular-based studies and even more so as a result of the multi-pronged assault freezing places on cancer cells. This molecular-level assault includes alterations in cellular pH, ionic concentrations and gradients (hypertonic during freezing, hypotonic during thawing), membrane fluidity, cellular dehydration, uncoupling of metabolic pathways, waste accumulation, protein unfolding/misfolding, and cytoskeletal disruption among others [Baust et al., 2013, 2014a, 2015; Baust, 2014]. Each of these individually has been shown to induce molecular-based cell death, whether apoptosis, secondary necrosis, autophagy, or some other form of cell death.
Further complicating this analysis is the fact that in addition to numerous initiation stresses, freezing has been shown to activate at least three individual apoptotic pathways including membrane, mitochondrial and nuclear induced apoptosis [Baust et al., 2010a; Clarke et al., 2004, 2007a, 2007b; Robilotto et al., 2013]. Many of the induction pathway studies have gone hand in hand with cryoadjunctive or cryosensitization research (detailed below). For instance, Clarke et al [2004] found a significant alteration in Bcl-2 and Bax mitochondrial protein levels following freezing resulting in a shift in mitochondrial signaling. This study further demonstrated that the utilization of Taxotere resulted in a further pro-death shift in mitochondrial signaling when combined with mild freezing resulting in enhanced prostate cancer cell death. Studies by Goel et al. [2007, 2009], Han et al. [2007], Jiang et al. [2008] and Baust et al. [2010a] also found the involvement of membrane mediated apoptosis in cell death following freezing. Mechanistically, Clarke et al. [2007b] showed the activation of caspase 8 (membrane mediated apoptosis) following freezing which could be amplified by pretreatment with TRAIL. A study by Klossner et al. [2008] reported on the differential sensitivity of varying forms of prostate cancer (early and late stage) to freezing injury via the activation of apoptosis. Specifically, this study found that early stage androgen sensitive prostate cancer cells (LNCaP) were more sensitive to freezing injury and had increased levels of apoptosis compared to late stage androgen insensitive prostate cancer cells (PC3). This was further confirmed in a PC3 cell model in which the androgen receptor gene was transfected into PC3 cells resulting in the cells re-expressing the androgen receptor (PC3-AR). In this case, as with the LNCaP cells, the PC3-AR cells demonstrated a higher degree of sensitivity to freezing and increased apoptotic activity post-freeze. Similarly, when the LNCaP cell model was “aged” in vitro they lost AR expression which resulted in the LNCaP-HP population being more resistant to freezing injury with a reduction in apoptosis, similar to that of the PC3 cells. Additional mechanistic studies by Baust et al. [...