
eBook - ePub
Sodium Dithionite, Rongalite And Thiourea Oxides: Chemistry And Application
Chemistry and Application
This is a test
- 244 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Sodium Dithionite, Rongalite And Thiourea Oxides: Chemistry And Application
Chemistry and Application
Book details
Book preview
Table of contents
Citations
About This Book
Sodium Dithionite, Rongalite and Thiurea Oxides provides an in depth overview of historical and newly developed fields of application for important sulfur-containing reductants. It begins with an introduction into the structure and general prope
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Sodium Dithionite, Rongalite And Thiourea Oxides: Chemistry And Application by Sergei V Makarov, Attila K Horv??th;Radu Silaghi-Dumitrescu;Qingyu Gao; in PDF and/or ePUB format, as well as other popular books in Biological Sciences & Science General. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Introduction
Sodium dithionite (Na2S2O4), sodium hydroxymethanesulfinate (rongalite) (HOCH2SO2Na) (HMS) and thiourea dioxide ((NH2)2CSO2) (TDO) have long been used in chemistry and chemical technology as reductants [1]. Recently, new fields of their application have been developed: reduction of graphene [2–8] and graphite oxides [9], synthesis of metal sulfides [10,11] and nanometer metal powders [12], metallization of fibers [13], organic synthesis [1,14,15] including organocatalytic reactions [16–22] and finally nonlinear phenomena in chemical kinetics [1]. Many newly discovered “relatives” of these compounds have been synthesized [1]. In the past few years, new and important information has been published on dithionite, particularly on the synthesis of complexes with uranium and f-elements as well as with decamethylsamarocene [23–25]. Other notable examples include the studies on photoisomerization of photochromic dithionite complexes in chiral crystals [26–30] and synthesis of the first polythionite [31]. It is found that to receive stable polythionite salt, a countercation should be sufficiently large. The same conclusion has been drawn for sulfoxylates [32]. It is shown that the most convenient precursor for synthesis of sulfoxylates in aqueous solution is thiourea dioxide.
The year 1870 may be considered as a starting point of the development of the chemistry of the above-mentioned sulfur-containing reducing agents. In that year, Schützenberger on the basis of Schönbein’s early observations [33] successfully prepared sodium dithionite for the first time [34,35]. To obtain even more stable reductants, later, in the end of 19th century as well as in the beginning of 20th century, a few α-hydroxyalkanesulfinates (including the most important sodium hydroxymethanesulfinate) were synthesized [36]. In 1910, TDO was prepared from the direct reaction of thiourea and hydrogen peroxide [37].
The careers of many eminent chemists were closely related to these chemicals. As a demonstrative example, the topic of the thesis of Nobel laureate Ernst Otto Fischer was “Mechanisms of reactions of carbon monoxide with nickel salts in the presence of dithionites and sulfoxylates” [38]. This thesis, under the direction of the famous German scientist Walter Hieber, a pioneer in the study of metal carbonyls, has become a significant contribution to the chemistry of sulfur-containing reducing agents. Later in this book, we shall also meet other famous chemists involved in the chemistry of these compounds.
Though using dithionite, hydroxymethanesulfinate and TDO as strong reducing agents remains the main field of their application, other important trends should also be highlighted. For example, in contrast to the redox reactions in which the sulfur-containing part of TDO plays the governing role, it is the nitrogen-containing part of TDO and trioxide (TTO) that determines their reactivity in the synthesis of guanidines and its derivatives [39]. The other important field is the synthesis of sulfones, where sulfur-oxygen fragments from dithionite, hydroxymethanesulfinate and TDO are embedded in the target compound [40].
And, of course, TDOs and TTOs deserve a special attention as they can be considered intermediates of the oxidation of thiourea and that of substituted thioureas in some processes. Indeed, the role and significance of thioureas have increased dramatically in the last years as a result of their versatile usage in supramolecular chemistry [41] and organocatalysis [42]. Different thioureas are now among the main environmentally friendly organocatalysts. The existence of two or three oxygen atoms capable of forming additional hydrogen bonds can improve catalytic properties of thiourea oxides [16–22]. However, in contrast to thiourea-catalyzed reactions, the mechanistic features of TDO-catalyzed reactions have been much less studied. TTO has not been used as an organocatalyst at all.
Unfortunately, there is only one relatively old review devoted to all these important sulfur-containing reductants with C–S and S–S bonds: dithionite, rongalite (hydroxymethanesulfinate) and TDO [1]. Since then, one short review on these compounds was published in 2013 (dithionite [43]), one in 2012 (rongalite in organic synthesis [14]), and an additional one about the product of their decomposition sulfoxylic acid [44]. In 2014, we also published a minireview on thiourea oxides [45]. It should be noted that the above-mentioned reviews, besides review on the application of rongalite in organic synthesis, are relatively short ones. There is no comprehensive and comparative review or book on the chemistry and application of these compounds. We believe that there is room for such a book and certainly hope that it will be interesting for a wide audience, especially for specialists in sulfur chemistry, coordination chemistry, biochemistry, nonlinear chemical kinetics, researchers of graphene and graphite, synthetic organic chemists, specialists in applied chemistry (paper, textile, polymer industries) and even specialists in building materials [46–48] and in history of chemistry.
Chapter 2
Synthesis
2.1Dithionites
Among all sulfur-containing reductants discussed in this book, sodium dithionite was synthesized first. In 1854, a famous chemist, the author of many unexpected observations [49,50], Christian Friedrich Schönbein reported that an aqueous solution of SO2 in contact with zinc turned rapidly yellow, meanwhile it was also capable of decolorizing a solution of indigo and litmus [33]. After a short period of time the solution deposited sulfur and lost its activity. Later, in 1860s French physician and chemist Paul Schützenberger (in a paper [51] authored by de Vries et al., his initials were falsely indicated as M. P. Since this paper was written in French, M thus stands for Monsieur as J. Wisniak (Ben-Gurion University) kindly paid our attention to this mistake) tried to isolate an active compound, but his experiments with SO2 and zinc were unsuccessful because the decolorizing power was lost very rapidly [35]. Better results were obtained when the SO2 (sulfurous acid) solution was replaced by concentrated solution of sodium bisulfite. In this case the reducing power was stronger and lasted for substantially more time, if the solution was kept out of contact with air. Schützenberger named the active substance sodium hydrosulfite and assigned an erroneous formula NaHSO2 • H2O [51]. Anyway, discovery of the strong reductant now having named sodium dithionite was among Schützenberger’s most valuable achievements and is mentioned in all related textbooks [49].
BASF was the first company to produce sodium dithionite in powder form, in 1906, by zinc dust process [52]. Zinc reacted with sulfur dioxide (bisulfite) in aqueous solution to produce zinc dithionite, which was transformed to sodium dithionite by adding sodium hydroxide. The main reactions are as follows:
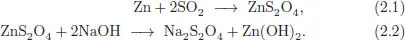
The product obtained was named Hydrosulfite (probably after Schützenberger) Conc. BASF [53]. The name “hydrosulfite” conveys the mistaken notion that the compound contains hydrogen. By the time this mistake was recognized, the brand name “Hydrosulfite Conc. BASF” had become so well-known commercially that the company decided not to change the name.
Later on, a process using sodium amalgam was developed. After that BASF...
Table of contents
- Cover
- Halftitle
- Title
- Copyright
- Acknowledgment
- Glossary
- Contents
- List of Figures
- List of Table
- About the Authors
- 1. Introduction
- 2. Synthesis
- 3. Structure
- 4. General Properties and Analysis
- 5. Stability in Solutions under Anaerobic and Aerobic Conditions
- 6. Organic Reactions
- 7. Inorganic Reactions and Material Chemistry
- 8. Industrial Applications
- 9. Miscellaneous
- Bibliography
- Index