
- 200 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About This Book
-->
Devoted to a diverse group of solid state scientists, the book has two objectives, both relating to structural chemistry: (i) a progressive analytic familiarization with the main parameters that govern the organization of crystallized matter and related crystal structures, (ii) a study of what are the various ways to 'read' a structure far beyond its representation in scientific articles. Hence, the reader will, from numerous examples illustrated in color, analyze what are the main characteristics of these structures, from their geometric characteristics, their coordination polyhedra, their connections with the resulting dimensionalities of these solids, including also the defects they exhibit, before looking at possibilities to classify structures, within which recurrence laws can emerge.
Chemists are required to understand the potentials of a new structure for becoming future materials scientists. The first part of the book is by no means a database for known structures, but facilitates a progressive understanding of the organization of the solid state. With these tools in hand, the reader is invited in the later part of the book to analyze new structures, and to also use new concepts for viewing structures in a more synthetic way for the future. Such new vision is already leading to the creation of completely new solids with outstanding characteristics that find applications in societal problems concerning energy, energy savings, environment and health.
The content is not exclusively academic but relates to the creation of innovative materials, through a more physical approach, that might condition the future of materials.
-->
Request Inspection Copy
-->
0 Readership: Undergraduate and graduate students in solid state sciences, coordination chemists and physicists.
0
- Presents a new accessible way for students and young researchers to understand solid state in a pedagogical way
- Detailed illustrations in color facilitates clarity and comprehension
Frequently asked questions
Information
Chapter 1
A First Familiarization
with Geometric Shapes:
The Dense Packings

The Koran (Surah 7, Verse 179)
1.1The Archimedian Polyhedra
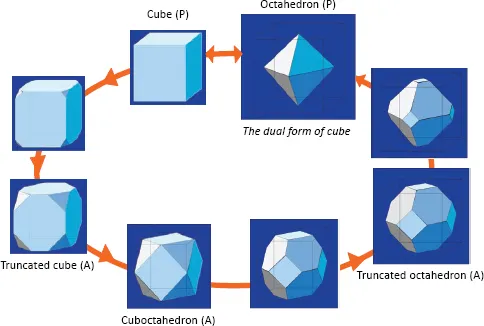
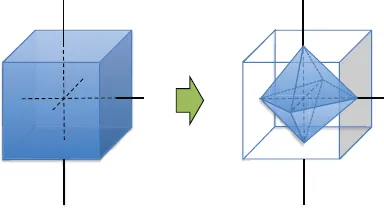
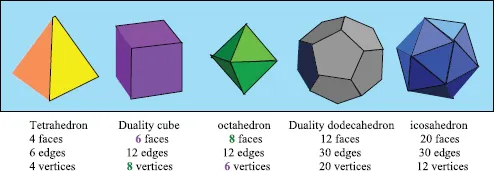


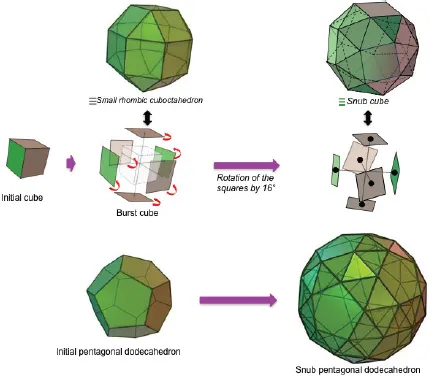

Table of contents
- Cover Page
- Title
- Copyright
- Dedication
- Preface
- Historical Introduction
- Chapter 1 A First Familiarization with Geometric Shapes: The Dense Packings
- Chapter 2 Some Notions of Symmetry
- Chapter 3 The Projections Their Reading, Their Uses…
- Chapter 4 Some Disorder within Order! Tiltings, Vacancies and Supercells
- Chapter 5 Armatures, Gilders and Bricks!… Other Ways of Reading a Structure
- Chapter 6 The Structural Relations … Their Deciphering
- Chapter 7 Crystal Chemistry? A Tool for Creation… or… The New Architects of Matter
- Epilogue
- Appendix 1 The Geometrical Characteristics of Platonic and Archimedian Polyhedra
- Appendix 2 The Exponential Scale, or the Mathematical Nature of Polyhedra
- Bibliography
- Index