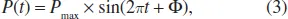1.What is the Sonochemistry?
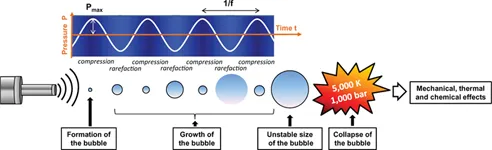
The term “sonochemistry” is used to describe the chemical and physical processes occurring in solution through the energy brought by power ultrasound.1–3 The effects of ultrasound are the consequence of the cavitation phenomenon, which is the formation, the growth and the collapse of gaseous microbubbles in liquid phase (Figure 1).3–5 Ultrasound is propagated through a series of compression and rarefaction waves in the liquid medium. When the acoustic power is sufficiently high, the rarefaction cycle exceeds the attractive forces of the molecules of the liquid and cavitation bubbles of a few micrometers in diameter are formed. Small amounts of vapor or gas from the medium enter in the bubble during its expansion phase and is not fully expelled during compression phase. The bubbles grow over the period of a few cycles to an equilibrium size for the particular frequency applied. The intense local effects (mechanical, thermal and chemical) due to the sudden collapse of those bubbles are at the origin of all the applications of sonochemistry.6–8
In water, at an ultrasonic frequency (f) of 20 kHz, each cavitation bubble collapse represents a localized hot-spot, generating temperatures of about 5,000 K and pressures superior to 1,000 bars (Figure 1). Many factors can affect the cavitation and the results of a sonochemical reaction: the acoustic power, the frequency, the hydrostatic pressure, the nature and the temperature of the solvent, the used gas and even the geometry of the reactor.9–13 The potential of sonochemistry is often directly connected to the choice of the sonochemical parameters or experimental conditions.
Figure 1: Schematic representation of the acoustic cavitation phenomenon.
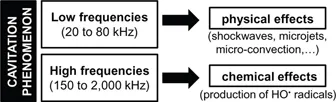
For example, the frequency is an essential parameter (Figure 2). Indeed, even if the whole mechanism is not elucidated yet, it is usually accepted that, in water, low frequencies (20–80 kHz) lead preferentially to physical effects (shockwaves, microjets, microconvection, etc.). On the contrary, high ultrasonic frequencies (150–2,000 kHz) favor the production of hydroxyl radicals (HO•) through the local hot-spots produced by cavitation, mainly leading to chemical effects. In a broad outline, it is possible to identify two great families of power ultrasound applications in chemistry based either on sonophysical effects or sonochemical effects. Conditions obtained in a medium submitted to ultrasound are accountable for a large number of physico-chemical effects as increase in kinetics of chemicals reactions, changes in reaction mechanisms, emulsification effects, erosion, crystallization, precipitation, etc.14,15
Figure 2: Predominant effects in water as a function of the frequency range.
The design of organic reactions, material preparations or other chemical processes under ultrasound requires a rigorous methodology and the complete report of all sonochemical parameters and experimental details. This latter point is essential for two reasons: (1) based on the available literature, the comparison between the obtained results and the wide spread of mechanistic explanations is not always trivial; (2) in the absence of these precautions, it could be really difficult (and even impossible in many cases) to reproduce an experiment from the literature.16
2.A Chemistry Based on Ultrasonic Waves
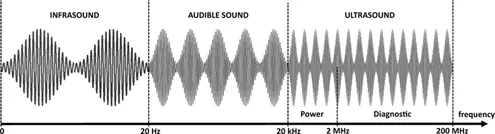
By definition, an ultrasonic wave is a sound wave belonging to the range between 20 kHz and 200 MHz that can be subdivided into two distinct regions: power ultrasound and diagnostic ultrasound (Figure 3). At lower frequencies, greater acoustic energy can be generated to induce cavitation in liquid medium (sonochemistry). Ultrasonic frequencies above 2 MHz do not produce cavitation. This range is particularly used in medical imaging (diagnostic ultrasound).
The sound is a wave generated by a mechanical vibration that travels due to the elasticity of the surrounding environment through longitudinal waves (straight line).
The sound waves do not propagate in vacuum.
The sound propagation is an elastic deformation (reversible): there is no transport of material.
Figure 3: Frequency ranges of sound.
To be described, a sound wave is often considered as a sinusoidal plane wave which is particularly characterized by the following properties:
•The frequency (f) in Hertz (Hz) or its inverse, the period (T) in seconds (s):
•
The
wavelength (
λ) usually expressed in nanometers (nm) or its inverse, the
wave number (
) in cm
−1:
•The alternative pressure wave is also characterized by its amplitude (P). The temporal evolution of the amplitude (P(t)) follows the simplified Eq. (3):
where Pmax is the maximal amplitude, t is the time and ϕ is the phase.
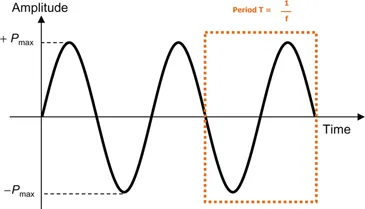
Figure 4 schematically represents the wave with the evolution of the amplitude as a function of the time. We do not give more details on the determination/calculation of the parameters such as amplitude or phase since they should not be very useful for the chemist who wants to develop sonochemical applications.
Figure 4: Representation of the sinusoidal plane wave of sound.
•The sound pressure or acoustic pressure, expressed in Pascal (Pa), is the local pressure deviation from the ambient pressure caused by a sound wave. It could be measured using a microphone in air o...