
Crystal Growth For Beginners: Fundamentals Of Nucleation, Crystal Growth And Epitaxy (Third Edition)
Fundamentals of Nucleation, Crystal Growth and Epitaxy
- 632 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Crystal Growth For Beginners: Fundamentals Of Nucleation, Crystal Growth And Epitaxy (Third Edition)
Fundamentals of Nucleation, Crystal Growth and Epitaxy
About This Book
-->
The processes of new phase formation and growth are of fundamental importance in numerous rapidly developing scientific fields such as modern materials science, micro- and optoelectronics, and environmental science. Crystal Growth for Beginners combines the depth of information in monographs, with the thorough analysis of review papers, and presents the resulting content at a level understandable by beginners in science. The book covers, in practice, all fundamental questions and aspects of nucleation, crystal growth, and epitaxy.
This book is a non-eclectic presentation of this interdisciplinary topic in materials science. The third edition brings existing chapters up to date, and includes new chapters on the growth of nanowires by the vapor–liquid–solid mechanism, as well as illustrated short biographical texts about the scientists who introduced the basic ideas and concepts into the fields of nucleation, crystal growth and epitaxy. All formulae and equations are illustrated by examples that are of technological importance. The book presents not only the fundamentals but also the state of the art in the subject.
Crystal Growth for Beginners is a valuable reference for both graduate students and researchers in materials science. The reader is required to possess some basic knowledge of mathematics, physics and thermodynamics.
--> --> Contents: Crystal–Ambient Phase Equilibrium;Nucleation;Crystal Growth;Epitaxial Growth;The Shoulders on Which We Stand; --> -->
Readership: Professionals and graduate students in materials science, dealing with crystals; with some basic knowledge of mathematics, physics and thermodynamics.
-->Nucleation, Epitaxy, Crystal Surfaces, Surface Structure, Crystal Growth, Misfit Dislocations, Thin Solid Films, Wetting, Surface Diffusion, Surfactanr Epitaxy, Nanowires, Ehrlich-schwoebel Effect, Silicon0
Frequently asked questions
Chapter 1
Crystal–Ambient Phase Equilibrium
1.1Equilibrium of Infinitely Large Phases

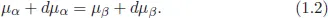

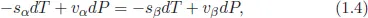


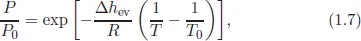
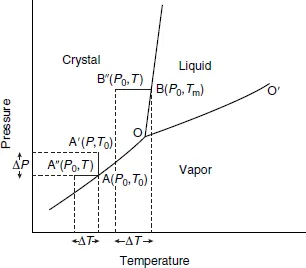
1.2Supersaturation
Table of contents
- Cover
- Halftitle
- Title
- Copyright
- Dedication
- Preface to the Third Edition
- Preface to the Second Edition
- Preface to the First Edition
- About the Author
- Contents
- Chapter 1. Crystal–Ambient Phase Equilibrium
- Chapter 2. Nucleation
- Chapter 3. Crystal Growth
- Chapter 4. Epitaxial Growth
- Chapter 5. The Shoulders on Which We Stand
- Appendix A. Units, Conversion Factors and Constants
- References
- Index