![]()
IV
Immunosorbent
![]()
Chapter 20
Immunoadsorbent in Hemoperfusion
Shenqi Wang*, Yaoting Yu†‡, and
Yanmiao Fan*
1.Introduction
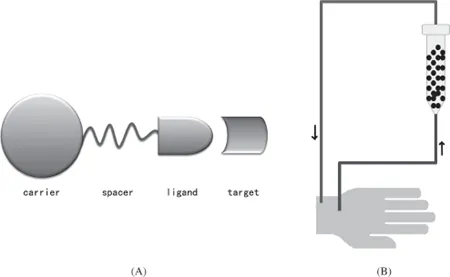
Sorbent perfusion is a novel approach to blood purification which can specifically remove endogenous and exogenous pathogenic toxins from the blood of patients (Behim et al., 1989). The technique involves passing whole blood or plasma of the patient through a cartridge filled with an adsorbent which can easily absorb the toxin or pathogenic molecules in the circulating blood. This approach has been developed in the past to be one of the most important areas in hemoperfusion, especially in clinical performances. The adsorbent is the core technique of the device and is composed of a carrier, spacer and ligand (Fig. 20.1a,b). According to selectivity, adsorbents can generally be classified as broad spectrum, affinity adsorbents and immuno-adsorbents, of which the latter has the highest selectivity (Lupien et al., 1976; Sinitsyn et al., 1990; Maaskant et al., 1986; Kojima et al., 1992).
Fig. 20.1. Schematic diagram of sorbent-perfusion.
In hemoperfusion, broad spectrum adsorbents such as activated charcoal can adsorb all kinds of pathogens existing in blood or plasma without a definite selectivity. An immunoadsorbent is defined as a preparation of antigen attached to a solid support or antigen in an insoluble form which absorbs homologous antibodies from a mixture of immunoglobulins (Dorland’s Medical Dictionary for Health Consumers, 2007). It is based on the principle of antigen–antibody interaction, and is therefore highly specific. In general materials used for preparation of the carrier can be activated charcoal, natural or synthetic bioactive polymers in the form of beads, fibers and membranes. The pathogenic molecules in the blood of patients are absorbed by the adsorbent via hydrophilic (electro-static forces) or hydrophobic interactions. Macroporous resins usually show high adsorption capacities especially for the removal of high molecular weight or “middle molecule” toxins (Falkenhagen et al., 1999; Terman et al., 1977; Von Appen et al., 1996). An antibody (Ab), also known as an immunoglobulin (Ig), is a large Y-shaped protein (“Y”) produced by plasma cells that is used by the immune system to identify and neutralize foreign objects such as bacteria and viruses. The antibody recognizes a unique part of the foreign target, called an antigen (Murphy et al., 2011; Litman et al., 1993). Each tip of the “Y” of an antibody contains a paratope (a structure analogous to a lock) that is specific for one particular epitope (similarly analogous to a key) on an antigen, allowing these two structures to bind together with precision (Wikipedia public domain).
Yatzidis et al. used activated charcoal to treat patients with kidney failure in 1964. In order to prevent problems related to particle release and damage to blood cells, Chang modified the surface of activated charcoal with albumin-ultrathin polymeric membrane coating on the surface (1969) and treated patients with acute poisoning, renal failure and liver failure (Chang, 1975). Graf et al. were the first to show that immunoadsorbents could be employed to selectively remove antibodies from actively and passively immunized rabbits. Schenkein et al. (1971) extended Chang’s albumin binding method (1969) and developed an extracorporeal immunoadsorbent system in which bovine serum albumin (BSA) was immobilized on agarose and proved to selectively remove BSA antibodies from the circulation. Because of the fragility of the supporting matrix which led to the releasing of fine particles causing thrombosis and poor biocompatibility properties of the adsorbent, new and stable carriers were developed. In order to solve the abovementioned problems, Chang introduced the concept of artificial cells, which are not a specific physical entity but an idea involving the preparation of artificial structures of cellular dimensions for possible replacement or supplement of deficient cell functions (Chang 1964 to 2007; Chang et al., 1987; Chang and Poznansky, 1968; Chang and Malave, 1970).
Artificial cells have three significant characteristics: (1) The membrane of an artificial cell separates its contents from the outside environment; (2) the cell membrane is biocompatible, can be very thin, yet strong, allowing specified molecules to pass in and out, and has a large surface area; (3) artificial cells can contain the same biological materials as biological cells. All these characteristics solved the above problems in hemoperfusion. Next, Chang developed albumin-based artificial cells by binding albumin to the ultrathin collodion membrane of artificial cells, which improved their biocompatibility. Albumin coating was also applied to synthetic immunoadsorbents.
In the early ’90s, adsorbents linked with antibodies or receptors used to treat autoimmune diseases were only classified as immunoadsorbent (Agishi, 1996). Following the progress of hemoperfusion, various ligands such as amino acids, sulfonic, phosphoric groups linked to polymers, synthetic peptides and protein A were developed and classified as ligands of immunoadsorbents. At the same time, clinical performances of immunoadsorbents had been developed for the treatment of autoimmune diseases, modification of hyper-acute renal xenograft rejection, removal of anti-HLA antibodies in transplant candidates and treatment of familial hypercholesterolemia with monoclonal antibodies to remove low-density lipoproteins. Then, a large number of immunoadsorbents were produced and commercialized (Terman, 1980; Terman et al., 1979a; 1979b; Hakim et al., 1990; Wingard et al., 1991; Yang et al., 2004). Chang’s concept of the “artificial cell” played a significant role in the development of immunoadsorbents.
The aim of this chapter is to present a brief overview of immunoadsorbents in hemoperfusion, and the ligand’s name is used to classify and describe the R&D of the adsorbents.
1.1.Protein A as Ligand
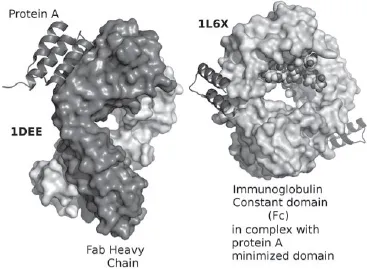
Protein A is a surface protein originally found in the cell wall of bacterium — Staphylococcus Aureus having a molecular weight of 56 kDa. Protein A is composed of five homologous Ig-binding domains that fold into a three-helix bundle. Each domain is able to bind proteins from many mammalian species, most notably IgGs (Graille et al., 2000; Idusogie et al., 2000). It binds the heavy chain within the Fc region of most immunoglobulins and also within the Fab region in the case of human VH3 family (Fig. 20.2).The immunoadsorbent of protein A is prepared by chemically linking the ligand to carriers such as agarose (sepharose) or silica beads via a spacer and is used for the removal of immunoglobulins in autoimmune diseases (Alkana et al., 2010; Samuelsson et al., 1999; Freiburghaus et al., 1988; Gjorstrup et al., 1991; Matic et al., 2000; 2001). Prosorba, the immunoadsorbent with protein A as ligand, was manufactured by Fresenius Hemocare (Redmont, CA, U.S.A.) and Immunosorba by Fresenius Hemocare (St. Wendel, Saarland, Germany). Both columns have been used clinically for the treatment of rheumatoid arthritis (Kutsuk et al., 1998). It is important to point out that Prosorba immunoadsorbent encountered some unprecedented challenges and difficulties as a treatment in clinical therapy because commercial production of the column has been ceased by the FDA since 2006 (Ward et al., 2011; Sanchez et al., 2013), and its treatment was not available in recent years. The main problems encountered are as follows: A considerable amount of protein A could be released from the matrix, and such contamination would not be tolerated in clinical applications; it was shown that Protein A is a B-cell super-antigen and postulated to be evolved in staphylococci as a means to impair antibody-mediated defences in the host where the staphylococcus is invading (Goodyear et al., 2003; Ward, 2011).
Fig. 20.2. Structure of a domain of protein A as a three-helix bundle binding to the heavy variable chain of a VH3 human Fab, left. Minimized protein A bound to Fc fragment of Rituximab. (Wikipedia, public domain.)
Super-antigens (SAgs) are a class of antigens which cause non-specific activation of T-cells resulting in polyclonal T cell activation and massive cytokine release (Llewelyn et al., 2002). This cytokine is closely linked with induction of autoimmunity (Stauffer et al., 2001), and the autoimmune Kawasaki Disease is known to be caused by SAgs infection (Arcus et al., 2000). The contact of patient’s blood with staphylococcal protein A may have an immunosuppressive effect caused by pharmacological mechanism rather than by an apheresis mechanism (Ward, 2011). Some leakage of Staphylococcus protein A (SPA) from the column (100–200 mg) into the patient’s blood during apheresis has been described, and many of the patients formed anti-SPA antibodies (Sasso et al., 2001). High cost of protein A adsorbent and low storage stability are also challenges for this adsorbent.
1.2.Tryptophane as Ligand
Tryptophane is one of the 22 standard amino acids that are essentially important in human diet. Due to the hydrophobicity property of the indole ring in the tryptophane molecule (Fig. 20.3), it is conducive to interact with acetylcholine receptor (AChR) and can be easily absorbed by the adsorbent. In 1982, Yamazaki et al. developed an adsorbent by linking tryptophane to PVA beads for the treatment of MG patients. Clinical performances demonstrated that the adsorbent can effectively remove AChR from patient’s plasma and alleviate MG syndrome. In 2005, Yu and Yang et al. synthesized immobilized tryptophan cellulosic bead type adsorbent and evaluated its adsorption capacity for binding AChR in whole blood. Experimental autoimmune myasthenia gravis (EAMG) rabbits were induced by Ta183-200 peptide, and extracorporeal whole blood hemoperfusion was conducted. Experimental results indicated that whole blood perfusion is an effective and safe approach in treating passive autoimmune myasthenia gravis by improving clinical manifestation, neuromuscular transmission function, enhancing the quantity of neuromuscular junction and reducing antibody titer. Bronchial asthma is a tracheal overreacted disease stimulated by antigenic reaction, conducted by the antibody of immunoglobulin E. At present, the treatment of bronchial asthma is mainly by medicine; immunoadsorption by extracorporeal hemoperfusion is under investigation. In 2008, Wang and Yu et al. synthesized agar gel adsorbent with tryptophan as ligand for the removal of immunoglobulin E (IgE) in bronchial asthma. In vitro results indicated the adsorption percentage of IgE in whole blood was 70.73% (Wang et al., 2008). Blood compatibility of the adsorbent was satisfactory and has a high potential to be used for whole blood purification in removal of IgE in bronchial asthma patients.
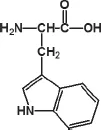
Fig. 20.3. Structure of tryptophan.
1.3.Phenylanaline as Ligand
Phenylanaline is an •-amino acid with the formula C6H5CH2CH(NH2)COOH (Fig. 20.4). The non-polar characteristic of this essential amino acid makes it widely used to absorb hydrophobic proteins.