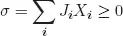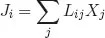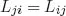![]()
Chapter 1
Scope
The aim of this book is to present the essence of non-equilibrium thermodynamics and its use for engineers. The field was established in 1931 and developed during the forties and fifties for transport in homogeneous phases. Applications of the theory are now increasing. Some perspectives on the applications are given after a brief introduction.
Non-equilibrium thermodynamics describes transport processes in systems that are not in global equilibrium. The field resulted from efforts of many scientists to find a more explicit formulation of the second law of thermodynamics. This already started in 1856 with Thomson’s studies of thermoelectricity, see [1]. Onsager is, however, counted as the founder of the field with his papers from 1931 [2, 3], see also [4], because these put earlier research by Thomson, Boltzmann, Nernst, Duhem, Jauman and Einstein into a systematic framework. Onsager was given the Nobel Prize in Chemistry in 1968 for this work.
The second law is formulated in terms of the entropy production σ. In Onsager’s formulation, the entropy production is given by the product sum of so-called conjugate fluxes, Ji, and forces, Xi, in the system. The second law then becomes
where σ is larger than or equal to zero. Close to equilibrium each flux is a linear combination of all forces,
and the reciprocal relations
apply. They now bear Onsager’s name. In order to use the theory, one first has to identify a complete set of extensive independent variables, αi. The resulting conjugate fluxes and forces are Ji = dαi/dt and Xi = (∂S/∂αi)αj≠i, respectively. Here t is the time and S is the entropy of the system. The three equations above contain, then, all information on the non-equilibrium behavior of the system.
Following Onsager, a consistent theory of non-equilibrium processes in continuous systems was set up in the nineteen forties by Meixner [5, 6, 7, 8] and Prigogine [9]. They calculated the entropy production for a number of physical problems. Prigogine received the Nobel prize for his work on dissipative structures in systems that are not in equilibrium in 1977, and Mitchell, the year after; for his application of the driving force concept to transport processes in biology [10].
The most general description of non-equilibrium thermodynamics is still the 1962 monograph by de Groot and Mazur [11]; reprinted in 1985 [12]. Haase’s book [13], also reprinted [14], contains many results for electrochemical systems and systems with temperature gradients. Katchalsky and Curran developed the theory for membrane transport and biophysical systems [15]. Their analysis was carried further by Caplan and Essig [16] and more recently by Demirel [17]. Førland and coworkers gave various applications in electrochemistry and biology, and they treated frost heave [18, 19]. Their book presented the theory in a way suitable for chemists. Newer books on equilibrium thermodynamics or statistical thermodynamics often include chapters on non-equilibrium thermodynamics, see e.g. [20]. In 1998, Kondepudi and Prigogine [21] presented an integrated approach of basic equilibrium and non-equilibrium thermodynamics. Jou et al. [22] published the second edition of their book on extended non-equilibrium thermodynamics and Öttinger gave a non-equilibrium description of the nonlinear regime [23].
Non-equilibrium thermodynamics is constantly being applied in new contexts. Fitts gave an early presentation of viscous phenomena [24]. Kuiken [25] has written the most general treatment of multi-component diffusion and rheology of colloidal systems. Rubi and coworkers [26, 27, 28] used the internal molecular degrees of freedom to explore the development within a system. We are now able to deal with the law of mass action [29] for chemical reactions within the framework of non-equilibrium thermodynamics [12] and shall do so in Chapter 7. Bedeaux and Mazur [30] extended the theory to quantum mechanical systems. Kjelstrup and Bedeaux [31] wrote a book dealing with transports into and across surfaces. Chapter 9 gives an introduction to this topic, and Chapter 10 provides examples of its use in membrane transport. These efforts have broadened the scope of the theory.
Chemical and mechanical engineering needs theories of transport in systems with gradients in pressure, concentration, and temperature, see Denbigh [32, 33]. In isotropic systems there is no coupling between tensorial (viscous) and vectorial (diffusional) phenomena, so the two classes can usually be dealt with separately [12]. We concentrate on isotropic systems in Chapters 4–7.
Simple vectorial transport laws have long worked well in engineering, but there is now an increased effort to be more precise. The need for more accurate flux equations in modeling [34] increases the need for non-equilibrium thermodynamics. The books by Taylor and Krishna [34], Cussler [35] and Demirel [17], which present Maxwell-Stefan’s formulation of the flux equations, are important books in this context. Krishna and Wesselingh [36] and Kuiken [25] have shown that the coefficients in the Maxwell-Stefan equations are relatively well-behaved, by analyzing an impressive amount of experimental data.
Non-equilibrium thermodynamics is necessary for a precise description of all systems that exchange heat, mass and charge. A frequent assumption is that there is a continuity in the intensive variables on the phase boundary. In Chapters 9 and 10, we show how this assumption can be lifted, taking the interface resistance into account.
There is a constant need in mechanical and chemical engineering to design systems that waste less work [37, 38, 39]. Fossil energy sources, as long as they last, lead to waste that may harm the environment. Better and more efficient use of energy resources is therefore central. It is then not good enough to only optimize the first law efficiency. The second law has also to be taken into account. The entropy production σ can be used to measure the efficiency of a technical process. Through non-equilibrium thermodynamics, one can develop methods to improve the second law efficiency. One purpose of the book is to present such methods, see Chapter 11.
The process industry may, in a not too distant future, be obliged to report not only on CO2 emission, but also on annual exergy destructed or entropy produced. Science Europe has proposed to introduce an exergy destruction footprint.1 Engineering companies are making first efforts to move in this direction, by including possibilities for exergy calculations in their process-modeling software. The public sector can enhance such a development, by giving benefits to those who limit their entropy production. The engineering community at large can develop further tools to accomplish this. Efforts in several fields, like control theory [40], are essential.
Non-equilibrium thermodynamics is the only theory that can be used to assess the second law efficiency in detail, or how valuable r...