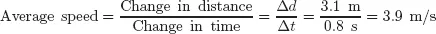![]()
Chapter 1
Concepts in Physics
“Those who aspire not to guess and divine, but to discover and know, who propose not to devise, mimic and fabulous worlds of their own, but to examine and dissect the nature of this very world itself, must go to facts themselves for everything.”
F. Bacon (1620)
It is impossible to undertake any serious study of nuclear chemistry without a working understanding of key aspects of physics and mathematics. On completion of this chapter and the associated questions, you should:
•Be familiar with Newton’s laws of motion and their relationship to work and energy.
•Be able to mathematically describe uniform circular motion and how it relates to the harmonic motion and properties of waves.
•Have an awareness of basic atomic structure and that unstable atoms gain stability through release of different types of radiation.
1.1Introduction to Classical Physics
Key Point: Classical physics is Newtonian physics which is built on macroscopic observations.
Much of what we consider to be “classical physics” stems from the work of Sir Isaac Newton (1642–1727), James Clerk Maxwell (1831–1879), and Ludwig Boltzmann (1844–1906) (Figure 1.1). Their theories on kinematics, electromagnetism, and thermodynamics, respectively, denominated the scientific landscape for much of the 19th and early 20th centuries. Understandably, these theories focused on the measurement of observable phenomena, such as an apple falling from a tree. To verify their theories, these scientists used a branch of mathematics known as calculus to quantify their observations. We will be making extensive use of calculus in our study of nuclear chemistry, especially when discussing the rate of radioactive decay. For this reason, we will revise many of the basic principles of calculus in Chapter 1.
Figure 1.1 The classical physicists (left to right): Sir Isaac Newton (1642–1727), James Clerk Maxwell (1831–1879), and Ludwig Boltzmann (1844–1906).
1.1.1Newton’s first law
Key Point: The change in an object’s position over time is given by the velocity of the object; the acceleration is the change in velocity over time.
To begin, consider Isaac Newton sitting beneath an apple tree in his garden at Woolsthorpe Manor in Lincolnshire. Newton thought about the rather obvious fact that an apple will remain attached to the tree unless something knocks/pulls it off. He realized that the same idea also applied to an object moving at constant speed — it would continue to move at that fixed speed unless some other force acted upon it. This forms the basis of Newton’s first law which was published in his Philosophiæ Naturalis Principia Mathematica in 1687. It was not without controversy and Newton’s ideas were famously rebuffed by Robert Hooke (1635–1703), a contemporary of Newton, whose competing work on gravitation was ultimately replaced by Newton’s theories.
To understand Newton’s first law, we must first introduce two terms which are easily confused: distance and displacement. To illustrate the difference, imagine Newton walking around the rectangular Great Court at Trinity College, Cambridge. If he walks 4 m east, then 2 m south, then 4 m west and 2 m north, he will have travelled a distance of 12 m. However, because he has ended up at his starting position, we say that his displacement is zero. This difference arises from the type of information conveyed by a measurement of distance or displacement. We say that distance is a scalar quantity — it only describes the magnitude of the distance travelled. Displacement is a vector quantity — it describes the difference between the initial and final points (we say it has magnitude and direction).
Now consider the famous incident of the apple falling from the tree. Suppose Newton timed how long it took an apple to fall from a branch 3.1m above ground level. If it took 0.8 s, we can calculate the average speed of the apple:
In this equation, the numerator, Δ
d, is read as “delta d” and this means the change in distance; similarly, the term in the denominator, Δ
t, is the change in time. Since we know both the magnitude and direction of the falling apple, we can replace the change in distance with change in displacement, Δ
s, and calculate the
average velocity,
, of the apple:
Notice the minus sign in front of the displacement and the velocity. This tells us that the displacement and resultant velocity are in the downward direction (velocity is therefore a vector quantity).
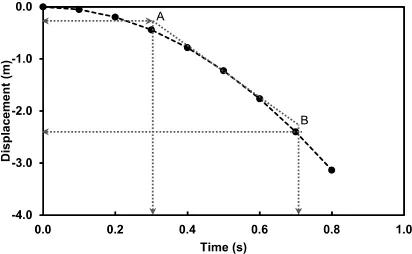
Now, suppose that Newton was able to measure the displacement of the apple every fraction of a second as it fell. Plotting a graph of displacement (y-axis) against time (x-axis) would produce something like that shown in Figure 1.2. Notice how the shape of the graph changes with time — it is not a straight line, but follows a curve known as a parabola. The significance of this is that the velocity of the apple is changing as it falls: at first, it falls relatively slowly (the curve is flatter), but as time progresses, it falls faster (the curve is steeper). When we use Δd/Δt or Δs/Δt to calculate the average velocity, this is not taken into account — it assumes a straight line (linear) relationship. If we wanted to know the velocity of the apple during the initial slower stage, we would have to use instantaneous velocity, v.
Suppose we wanted to calculate the instantaneous velocity at 0.5 s. First, we could draw a tangent, AB, to the point at t = 0.5s and the gradient of this tangent will equal the instantaneous velocity. For the tangent given in Figure 1.2, the gradient is given by:
Figure 1.2 Displacement–time graph for an apple in free fall. The gradient of the tangent, AB, can be used to determine the average velocity.
The accurac...