
- 836 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Nanomaterials for Energy Conversion and Storage
About this book
-->
The use of nanomaterials in energy conversion and storage represents an opportunity to improve the performance, density and ease of transportation in renewable resources. This book looks at the most recent research on the topic, with particular focus on artificial photosynthesis and lithium-ion batteries as the most promising technologies to date. Research on the broad subject of energy conversion and storage calls for expertise from a wide range of backgrounds, from the most fundamental perspectives of the key catalytic processes at the molecular level to device scale engineering and optimization. Although the nature of the processes dictates that electrochemistry is a primary characterization tool, due attention is given to advanced techniques such as synchrotron studies in operando. These studies look at the gap between the performance of current technology and what is needed for the future, for example how to improve on the lithium-ion battery and to go beyond its capabilities.
Suitable for students and practitioners in the chemical, electrochemical, and environmental sciences, Nanomaterials for Energy Conversion and Storage provides the information needed to find scalable, economically viable and safe solutions for sustainable energy.
-->
Contents:
- The Principle of Photoelectrochemical Water Splitting (Peiyan Ma and Dunwei Wang)
- Semiconducting Photocatalysis for Solar Hydrogen Conversion (Shaohua Shen and Jie Chen)
- Visible-Light-Driven Photocatalysis (Qingzhe Zhang, Yanlong Liu, Zhenhe Xu, Yue Zhao, Mohamed Chaker and Dongling Ma)
- Metal-Nitride Nanostructures: Emerging Catalysts for Artificial Photosynthesis (Md Golam Kibria, Bandar AlOtaibi and Zetian Mi)
- Surface Engineering of Semiconductors for Photoelectrochemical Water Splitting (Gongming Wang, Yi Yang and Yat Li)
- Photoanodic and Photocathodic Materials Applied for Free-Running Solar Water Splitting Devices (Miao Zhong, Hiroyuki Kaneko, Taro Yamada and Kazunari Domen)
- Electrocatalytic Processes in Energy Technologies (Yang Huang, Min Zeng, Qiufang Gong and Yanguang Li)
- Soft X-Ray Spectroscopy on Photocatalysis (Yi-Sheng Liu, Cheng-Hao Chuang and Jinghua Guo)
- Photoelectrochemical Tools for the Assessment of Energy Conversion Devices (Isaac Herraiz-Cardona and Sixto Gimenez)
- Fundamentals of Rechargable Batteries and Electrochemical Potentials of Electrode Materials (Chaofeng Liu and Guozhong Cao)
- Revitalized Interest in Vanadium Pentoxide as Cathode Material for Alkali-Ion Batteries (Yanwei Li, Jinhuan Yao, Robert C Massé, Evan Uchaker and Guozhong Cao)
- Tin-Based Compounds as Anode Materials for Lithium-Ion Storage (Ming Zhang and Guozhong Cao)
- Beyond Li-Ion: Electrode Materials for Sodium- and Magnesium-Ion Batteries (Robert Massé, Evan Uchaker and Guozhong Cao)
- Nanomaterials and Nanostructures for Regulating Ions and Electron Transport in Advanced Energy Storage Devices (Yu Wang and Wei-Hong Zhong)
-->
--> Readership: Students, researchers and practitioners in the chemical, electrochemical, and environmental sciences. -->
Keywords:Nanomaterials;Lithium-Ion Batteries;Electrochemistry;Energy Conversion;Energy Storage;Artificial PhotosynthesisReview:0
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
CHAPTER 1
THE PRINCIPLE OF PHOTOELECTROCHEMICAL WATER SPLITTING
Wuhan University of Technology, Wuhan, China
†Department of Chemistry, Boston College,
2609 Beacon St., Chestnut Hill, MA 02467, USA
‡[email protected]
§[email protected]
1.Introduction
1.1.General background
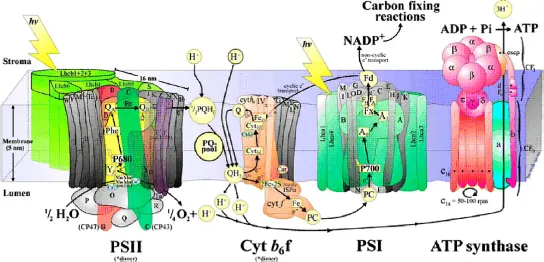
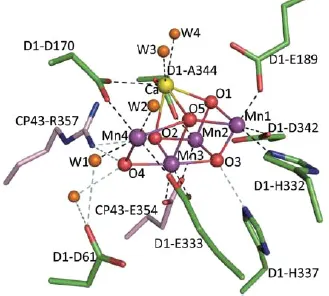

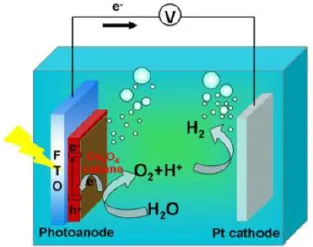
1.2.Fundamental considerations in selecting a material for solar water splitting
1.2.1.Potential requirement
1.2.2.Appropriate band structure
1.2.3.High crystallization and surface area
1.2.4.Stability
Table of contents
- Cover Page
- Title
- Copyright
- Preface
- About the Editors
- Contents
- Chapter 1. The Principle of Photoelectrochemical Water Splitting
- Chapter 2. Semiconducting Photocatalysis for Solar Hydrogen Conversion
- Chapter 3. Visible-Light-Driven Photocatalysis
- Chapter 4. Metal Nitride Nanostructures: Emerging Catalysts for Artificial Photosynthesis
- Chapter 5. Surface Engineering of Semiconductors for Photoelectrochemical Water Splitting
- Chapter 6. Photoanodic and Photocathodic Materials Applied for Free-Running Solar Water Splitting Devices
- Chapter 7. Electrocatalytic Processes in Energy Technologies
- Chapter 8. Soft X-ray Spectroscopy on Photocatalysis
- Chapter 9. Photoelectrochemical Tools for the Assessment of Energy Conversion Devices
- Chapter 10. Fundamentals of Rechargeable Batteries and Electrochemical Potentials of Electrode Materials
- Chapter 11. Revitalized Interest in Vanadium Pentoxide as Cathode Material for Alkali-Ion Batteries
- Chapter 12. Tin-Based Compounds as Anode Materials for Lithium-Ion Storage
- Chapter 13. Beyond Li Ion: Electrode Materials for Sodium- and Magnesium-Ion Batteries
- Chapter 14. Nanomaterials and Nanostructures for Regulating Ions and Electron Transport in Advanced Energy Storage Devices
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app