
- 392 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Drug Delivery Systems
About this book
-->
With the alarming increase in cancer diagnoses and genetic illnesses, traditional drug agents and their delivery media need to be re-evaluated to address a quickly evolving field. With newer smart materials for the controlled release of macromolecules, peptides, genetic material, etc. further complications arise, such as material performance, synthesis, functionalization and targeting, biological identity, and biocompatibility.
The book provides a comprehensive overview of the recent developments on "smart" targeting and drug delivery systems with a variety of carriers like nanoparticles, membranes, and hydrogels. It contains detailed descriptions on the recent trends in this field in the ongoing battle with catastrophic diseases like cancer. This field of research has been in its infancy and continues to face growth, and with it, further challenges and difficulties along the way toward maturity, which are accurately introduced in this book.
--> -->
Contents:
- Drug Delivery Systems: Possibilities and Challenges (Ryan Spitler, Saeid Zanganeh, Tahereh Jafari, Nasser Khakpash, Mohsen Erfanzadeh, Jim Q Ho, and Nastaran Sakhaie)
- Nanoparticles in Circulation: Blood Stability (Saeid Zanganeh, Tahereh Jafari, Nasser Khakpash, Mohsen Erfanzadeh, and Jim Q Ho)
- How do Nanoparticles (NPs) Pass Barriers? (Saeid Zanganeh, Ryan Spitler, Najme Javdani, and Jim Q Ho)
- Gated Porous Materials for Biomedical Application (Félix Sancenón, Erick Yu, Elena Aznar, M Dolores Marcos, and Ramón Martínez-Máñez)
- Controlled Release from Iron Oxide Nanoparticles (Masoud Rahman)
- The Reverse of Controlled Release: Controlled Sequestration of Species and Biotoxins into Nanoparticles (NPs) (Jenifer Gómez-Pastora, Eugenio Bringas, María Lázaro-Díez, José Ramos-Vivas, and Inmaculada Ortiz)
- Membranes for Controlled Release (Vida Araban, Neda Aslankoohi, and Mohammad Raoufi)
- Controlled Released from Hydrogel (Hossein Riahinezhad, Vida Araban, and Mohammad Raoufi)
- Nano Delivery Systems (Sophie Laurent, Afsaneh Lahooti, Saeed Shanehsazzadeh, and Robert N Muller)
- Legal Framework for Protection of Pharmaceutical Trade Marks in Europe and USA (Mohammad Hossein Erfanmanesh, and Shirin Sharifzadeh)
- Future Perspective on the Smart Delivery of Biomolecules (Erick Yu, Félix Sancenón, Elena Aznar, Ramón Martínez-Máñez, María Dolores Marcos, Mohammad J Hajipour, Morteza Mahmoudi, and Pieter Stroeve)
--> -->
Readership: Nanotechnologists; biomedical engineers; chemical engineers; materials scientists; biotechnology researchers; chemists; biological scientists; cell physiologists; medical scientists; gene therapists.
-->Keywords:Drug Delivery Systems;Nanoparticles;Biomaterials;TargetingReview: Key Features:
- Comprehensive overview on "smart" targeting and drug delivery systems
- Understanding of the biological identity of nanoparticles for drug delivery applications
- Detailed information on the legal framework for protection of pharmaceutical trade mark in Europe and the United States
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1
Drug Delivery Systems: Possibilities and Challenges
| I. | Introduction |
| II. | Liposomal and Targeted Drug Delivery System A.Evolution of liposomal drug targeting B.Liposome classification C.Types of liposomal drug delivery platforms D.Passive targeting 1.Limitations of passive targeting E.Active targeting F.Challenges in liposomal drug delivery |
| III. | Transdermal Drug Delivery A.Passive transdermal drug delivery B.Active transdermal drug delivery C.Transdermal delivery system design D.Transdermal drug delivery systems technologies 1.Polymer membrane partition-controlled TDD systems 2.Polymer matrix diffusion-controlled TDD systems 3.Drug reservoir gradient-controlled TDD systems 4.Micro-reservoir dissolution-controlled TDD systems E.Transdermal drug delivery challenges |
| IV. | Microemulsion Drug Delivery System A.Microemulsion in pharmaceuticals 1.Transdermal route 2.Nasal route 3.Oral route 4.Parenteral route 5.Ocular delivery 6.Tumor targeting 7.Cancer treatment B.Microemulsion challenges |
| V. | Nanotechnology for Drug Delivery Systems A.Polymers in drug delivery 1.Liposomes 2.Micelles 3.Dendrimers 4.Nanospheres and nanocapsules B.Inorganic nanoparticles 1.SPIONs C.Nanotechnology challenges |
| VI. | Concluding Remarks |
Abbreviations
CPP | cell penetrating peptides |
CRT | controlled-release technology |
DSPE | distearoylphosphatidylethanolamine |
DDS | drug delivery systems |
EPR | enhanced permeability and retention |
EMEA | European Medicine Evaluation Agency |
FDA | Food and Drug Administration |
LDL | low density lipoprotein |
LUV | large unilamellar liposomes |
mPEG | methoxy polyethylene glycol |
MNs | micro needles |
NPs | nanoparticles |
PEG-PE | poly(ethylene) glycol-phosphatidylethanolamine |
PEG | polyethylene glycol |
RES | reticuloendothelial system |
SPIONs | superparamagnetic iron oxide |
TDDS | targeted drug delivery system |
mAb | monoclonal antibodies |
TATp | transcription protein |
TDDS | transdermal drug delivery systems |
I.Introduction
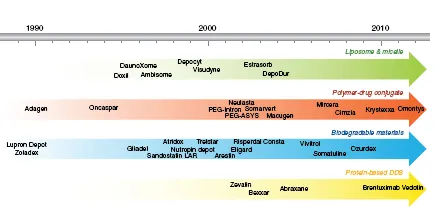
Table of contents
- Cover
- Halftitle
- Series Editors
- Title
- Copyright
- About the Editors
- Contents
- Chapter 1 Drug Delivery Systems: Possibilities and Challenges
- Chapter 2 Nanoparticles in Circulation: Blood Stability
- Chapter 3 How do Nanoparticles (NPs) Pass Barriers?
- Chapter 4 Gated Porous Materials for Biomedical >Applications
- Chapter 5 Controlled Release from Iron Oxide Nanoparticles
- Chapter 6 The Reverse of Controlled Release: Controlled Sequestration of Species and Biotoxins into Nanoparticles (NPs)
- Chapter 7 Membranes for Controlled Release Vida Araban, Neda Aslankoohi, and Mohammad Raoufi
- Chapter 8 Controlled Released from Hydrogel
- Chapter 9 Nano Delivery Systems Sophie Laurent, Afsaneh Lahooti, Saeed Shanehsazzadeh, and Robert N. Muller
- Chapter 10 Legal Framework for Protection of Pharmaceutical Trade Marks in Europe and USA
- Chapter 11 Future Perspective on the Smart Delivery of Biomolecules
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app